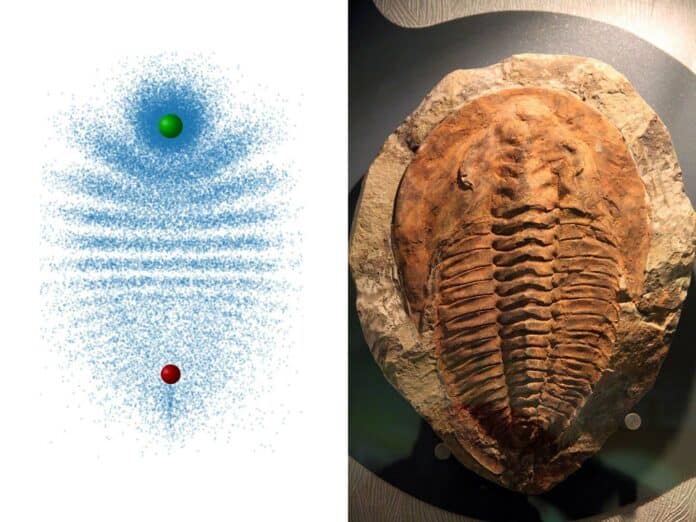
In trilobite Rydberg molecules, an atom in its usual state is connected to a Rydberg electron (an electron in an outer orbit) in a superposition of high angular momentum states. This creates a homonuclear molecule with a permanent electric dipole moment in the kilo-debye range.
Until now, scientists had only seen trilobite molecules only with admixtures of low-l states. Kaiserslautern physicists have succeeded for the first time in directly observing pure trilobite Rydberg molecules.
These molecules have a unique shape that looks like trilobite fossils, and they hold the record for having the biggest electric dipole moments among all known molecules. Scientists used a special device that can create these delicate molecules at extremely low temperatures. This helps us understand how they stick together, which is different from all other types of chemical bonds.
In their experiment, the physicists worked with a group of rubidium atoms cooled to an extremely low temperature of about 100 microkelvin, just 0.0001 degrees above absolute zero. After cooling, they used lasers to excite some of these atoms to a state known as the Rydberg state.
The outermost electron is moved to orbits far from the atomic nucleus during this process. The orbit’s radius can exceed one micrometer, making the electron cloud larger than a small bacterium. These highly excited atoms are also found in interstellar space, and they are extremely reactive chemically.
A ground state atom forms a molecule when it is situated within this large Rydberg atom. Unlike traditional chemical bonds such as covalent, ionic, metallic, or dipolar, trilobite molecules are bound by a completely different mechanism. Quantum mechanical scattering of the Rydberg electron from the ground state atom binds them together. Picture the electron swiftly orbiting the nucleus, colliding with the ground state atom on each round trip. Contrary to our intuition, quantum mechanics explains that these collisions effectively attract the electron and the ground-state atom.
These molecules have remarkable properties. Because of the wave nature of the electron, the collisions create an interference pattern resembling a trilobite. The bond length is as large as the Rydberg orbit, much bigger than any other diatomic molecule. The strong attraction between the electron and the ground state atom results in a huge permanent electric dipole moment exceeding 1700 Debye.
To observe these molecules, scientists designed a specialized vacuum apparatus. This tool enables the preparation of ultracold atoms through laser cooling and the subsequent detection of the molecules using spectroscopy.
Professor Herwig Ott, who researches ultracold quantum gases and quantum atom optics at the University of Kaiserslautern-Landau, said, “The findings contribute to understanding the fundamental binding mechanisms between ground state atoms and Rydberg atoms, which are increasingly promising for quantum computing applications. This discovery adds to our understanding of Rydberg systems, which can be both exotic and useful.”
Journal Reference:
- Althön, M., Exner, M., Blättner, R. et al. Exploring the vibrational series of pure trilobite Rydberg molecules. Nat Commun 14, 8108 (2023). DOI: 10.1038/s41467-023-43818-7