Another way to deal with dissecting and outlining new particle conductors — a key segment of rechargeable batteries — could quicken the advancement of high-vitality lithium batteries, and perhaps other vitality stockpiling and conveyance gadgets, for example, power modules, scientists say.
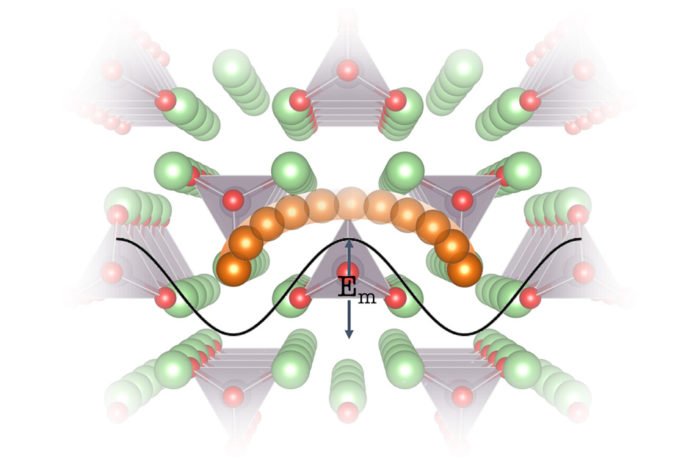
The new approach depends on understanding the way vibrations travel through the precious stone grid of lithium particle conductors and connecting that with the way they restrain particle movement. This gives an approach to find new materials with improved particle versatility, permitting fast charging and releasing.
In the meantime, the technique can be utilized to lessen the material’s reactivity with the battery’s anodes, which can abbreviate its valuable life. These two attributes — better particle versatility and low reactivity — have had a tendency to be fundamentally unrelated.
The new idea was created by a group led by W.M. Keck Professor of Energy Yang Shao-Horn, graduate understudy Sokseiha Muy, late graduate John Bachman Ph.D. ’17, and Research Scientist Livia Giordano, alongside nine others at MIT, Oak Ridge National Laboratory, and foundations in Tokyo and Munich. Their discoveries were accounted for in the diary Energy and Environmental Science.
The new plan rule has been around five years really taking shape, Shao-Horn says. The underlying reasoning began with the approach she and her gathering have used to comprehend and control impetuses for water part, and applying it to particle conduction — the procedure that lies at the core of rechargeable batteries, as well as other key advances, for example, power modules and desalination frameworks.
While electrons, with their negative charge, spill out of one shaft of the battery to alternate (accordingly giving energy to gadgets), positive particles stream the other path, through an electrolyte, or particle conductor, sandwiched between those posts, to finish the stream.
Normally, that electrolyte is a fluid. A lithium salt broke down in a natural fluid is a typical electrolyte in the present lithium-particle batteries. In any case, that substance is combustible and has some of the time made these batteries burst into flames. The scan has been on for a strong material to supplant it, which would take out that issue.
An assortment of promising strong particle conductors exist, however, none is steady when in contact with both the positive and negative cathodes in lithium-particle batteries. Hence, looking for new strong particle conductors that have both high particle conductivity and solidness is basic.
Be that as it may, dealing with the wide range of basic families and arrangements to locate the most encouraging ones is an exemplary needle in a sheaf issue. That is the place the new outline standard comes in.
The thought is to discover materials that have particle conductivity equivalent to that of fluids, however with the long haul solidness of solids. The group asked, “What is the key guideline? What are the plan standards on a general auxiliary level that represent the coveted properties?” Shao-Horn says. A mix of hypothetical investigation and trial estimations has now yielded a few answers.
Muy, the paper’s lead author said, “We realized that there are a lot of materials that could be discovered, but no understanding or common principle that allows us to rationalize the discovery process. We came up with an idea that could encapsulate our understanding and predict which materials would be among the best.”
The new idea was created by a group drove by W.M. Keck Professor of Energy Yang Shao-Horn, graduate understudy Sokseiha Muy, late graduate John Bachman PhD ’17, and Research Scientist Livia Giordano, alongside nine others at MIT, Oak Ridge National Laboratory, and organizations in Tokyo and Munich. Their discoveries were accounted for in the diary Energy and Environmental Science.
The new plan standard has been around five years really taking shape, Shao-Horn says. The underlying reasoning began with the approach she and her gathering have used to comprehend and control impetuses for water part, and applying it to particle conduction — the procedure that lies at the core of rechargeable batteries, as well as other key advancements, for example, energy components and desalination frameworks.
While electrons, with their negative charge, spill out of one shaft of the battery to alternate (in this way giving energy to gadgets), positive particles stream the other route, through an electrolyte, or particle conductor, sandwiched between those posts, to finish the stream.
Ordinarily, that electrolyte is a fluid. A lithium salt disintegrated in a natural fluid is a typical electrolyte in the present lithium-particle batteries. Yet, that substance is combustible and has once in a while made these batteries burst into flames. The look has been on for a strong material to supplant it, which would kill that issue.
An assortment of promising strong particle conductors exist, however, none is steady when in contact with both the positive and negative terminals in lithium-particle batteries, Shao-Horn says. Along these lines, looking for new strong particle conductors that have both high particle conductivity and steadiness is basic.
In any case, dealing with the wide range of basic families and creations to locate the most encouraging ones is an exemplary needle in a sheaf issue. That is the place the new outline guideline comes in.
The thought is to discover materials that have particle conductivity similar to that of fluids, yet with the long haul security of solids. The group asked, “What is the key guideline? What are the outline standards on a general basic level that oversee the coveted properties?” Shao-Horn says. A mix of hypothetical examination and test estimations has now yielded a few answers.
The paper is presented in the journal Energy and Environmental Science.