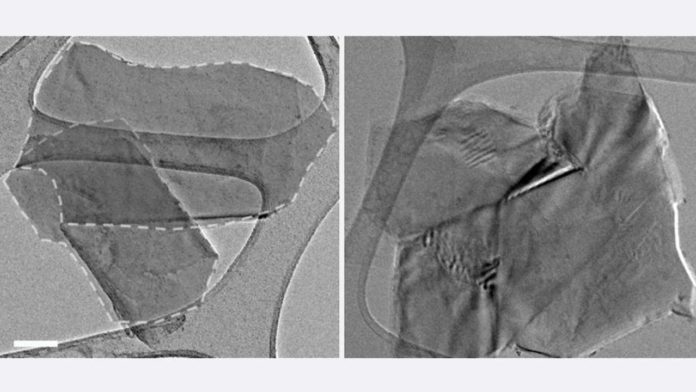
A new report offers insight on how artificially adjusted graphene powder can be squeezed into a lightweight, semi-porous strong that holds a large number of the solid and conductive characteristics of graphite, the type of carbon found in pencils, greases and numerous different items that ordinarily requires high-temperature handling to make.
Scientists demonstrated the environmentally friendly, scalable process can be done in minutes by hand by grinding chemically modified graphene into a powder and using a hand-powered press to squeeze the powder into a solid pellet.
Mohamad Kabbani, a former graduate student of Rice materials scientist Pulickel Ajayan and lead author of the paper.
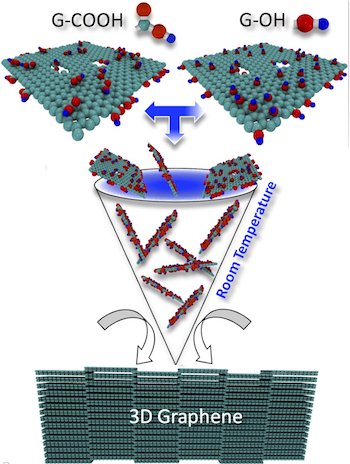
Kabbani already demonstrated how carbon nanotubes could be transformed into graphene with a mortar and pestle as opposed to cruel chemicals. This time, he and his associates showed how to make a battery-sized pellet, however, the graphene powders with compound functionalities appended to it can be squeezed into any frame.
Kabbani said the material could be reasonable for basic, synergist, electrochemical and electronic applications. This is the first time anyone’s made these at room temperature and without very high pressure. Usually, these kinds of materials require sintering (a process that uses pressure or heat to form solids without melting them) at temperatures above 1,000 degrees Celsius to produce strong pellets. In this case, mechano-chemistry at the nanoscale saved us a lot of energy and money.”
The procedure started with two arrangements of functionalized nanotubes, one with carboxylic corrosive and the other with hydroxyl particles. When squashed to consolidate them either by hand or machine, they are put in a lab-scale water driven press and subjected to 5 tons of weight. The useful groups cross-connected the graphene sheets to each other, and despite the fact that no fluids were included, they delivered a minor measure of water as a result of the response, Kabbani said.
The pellets stayed stable when put in heated water for five hours, notwithstanding when blended; this affirmed the interlocking of the graphene sheets inside, the analysts detailed.
Kabbani said. “The pellet material is stronger and lighter than commercial graphite electrodes and could be promising for electrical storage applications with high energy and power densities.”
Co-authors of the paper are postdoctoral researchers Vidya Kochat and Matias Soto; Chandra Sekhar Tiwary, a former postdoctoral researcher at Rice and now an assistant professor at IIT Gandhinagar, India; Sanjit Bhowmick and Syed Asif of Bruker Nano Surfaces, Minneapolis; Anirban Som, K.R. Krishnadas, and Thalappil Pradeep of the Indian Institute of Technology, Madras, India; Ahmad Kabbani of the Lebanese American University, Beirut; and Enrique Barrera, a professor of materials science and nanoengineering, and Robert Vajtai, an associate research professor of materials science and nanoengineering, both at Rice.
A report is published in the Journal Carbon.