Polycyclic aromatic hydrocarbons (PAHs) represent key molecular building blocks prompting carbonaceous nanoparticles recognized in combustion systems and extraterrestrial environments. Be that as it may, the comprehension of their formation and growth in these high-temperature environments has remained elusive.
In a new study, a team of scientists has produced a chain of ringed, carbon-containing particles by consolidating two highly reactive chemical species that are called free radicals since they contain unpaired electrons. Through this study, scientists showed how these chemical processes could prompt the advancement of carbon-containing graphene-type PAHs and 2D nanostructures.
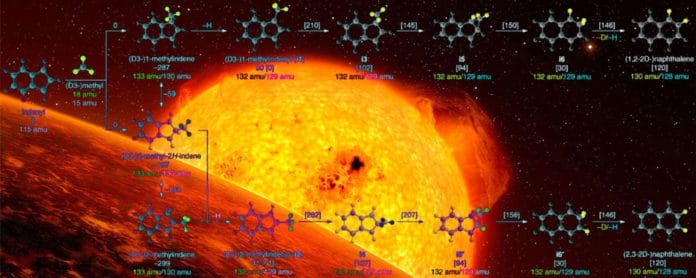
The investigation demonstrated an approach to associate a five-sided sub-atomic ring with a six-sided sub-atomic ring and to likewise convert five-sided molecular rings to six-sided rings, which is a stepping stone to a more extensive scope of enormous PAH molecules.
Musahid Ahmed, a scientist in Berkeley Lab’s Chemical Sciences Division said, “This is something that people have tried to measure experimentally at high temperatures but have not done before. We believe this is yet another pathway that can give rise to PAHs.”
Previous studies by the same research team have also identified a couple of other pathways for PAHs to develop in space. The studies suggest there could be multiple chemical routes for life’s chemistry to take shape in space.
“It could be all the above so that it isn’t just one,” Ahmed said. “I think that’s what makes this interesting.”
The experiments at Berkeley Lab’s ALS – which produces X-rays and other types of light supporting many different types of simultaneous experiments – used a portable chemical reactor that combines chemicals and then jets them out to study what reactants formed in the heated reactor.
Researchers used a light beam tuned to a wavelength known as “vacuum ultraviolet” or VUV produced by the ALS, coupled with a detector (called a reflectron time-of-flight mass spectrometer), to identify the chemical compounds jetting out of the reactor at supersonic speeds.
In this study, scientists combined chemical radicals CH3 (aliphatic methyl radical) with C9H7 (aromatic 1-indenyl radical) at a temperature of about 2,105 Fahrenheit degrees to ultimately produce molecules of a PAH known as naphthalene (C10H8) that is composed of two joined benzene rings.
Scientists noted, “The reactants produced from two radicals had been theorized but hadn’t been demonstrated before in a high-temperature environment because of experimental challenges.”
Ahmed said, “The radicals are short-lived – they react with themselves and react with anything else around them. The challenge is, ‘How do you generate two radicals at the same time and in the same place, in an extremely hot environment?’ We heated them up in the reactor, they collided and formed the compounds, and then we expelled them out of the reactor.”
Professor Ralf I. Kaiser at the University of Hawaii at Manoa said, “For several decades, radical-radical reactions have been speculated to form aromatic structures in combustion flames and in deep space, but there has not been much evidence to support this hypothesis. The present experiment clearly provides scientific evidence that reactions between radicals at elevated temperatures do form aromatic molecules such as naphthalene.”
The method used in the study can be used to determine how specific types of chemical compounds form in space.
Scientists at Berkeley Lab, the University of Hawaii at Manoa, and Florida International University participated in this study. The work was supported by the U.S. Department of Energy Office of Science’s Basic Energy Sciences program and a Presidential Fellowship at Florida International University.
The study is published in Nature Communications.