Lead-based perovskites are quite promising in applications of large-scale photovoltaic technology. However, toxicity is one of the crucial issues in these materials.
In the search for Lead-free perovskite, UNIST scientists have taken a major step forward toward a new generation of solar cells. They have developed new perovskite material that works as a charge regenerator with dye‐sensitized solar cells and have higher efficiency and stability.
Scientists used the vacancy‐ordered double perovskite (Cs2SnI6). They primarily examined the charge transfer mechanism of Cs2SnI6 with the aim of clarifying the function of its surface state.
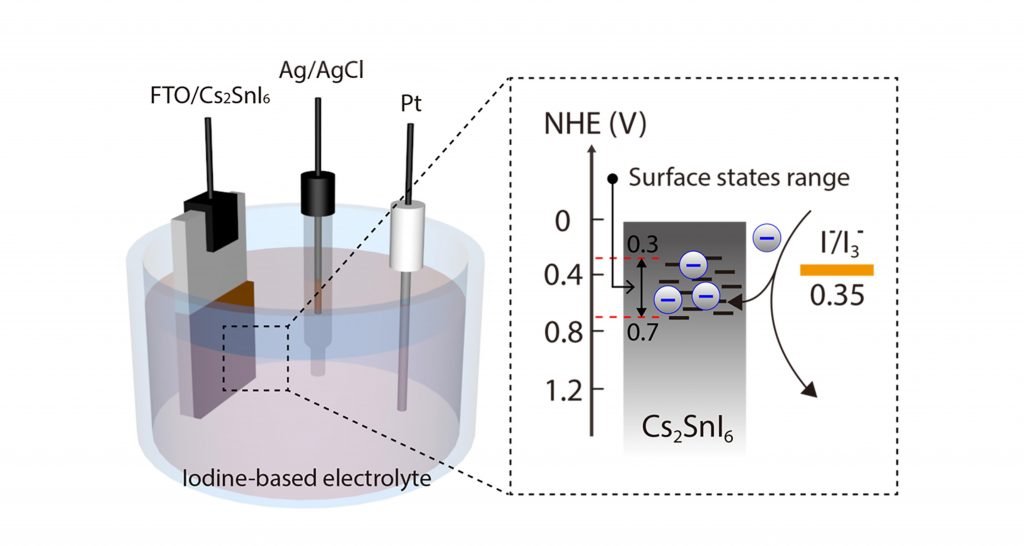
For this reason, a 3‐electrode system was produced to observe charge exchange through the surface state of Cs2SnI6. Cyclic voltammetry and Mott– Schottky investigations were additionally used to test the surface state of Cs2SnI6, whose potential is identified with its bandgap.
Until now, the surface states of Cs2SnI6 and their function remain largely unclear. But, through this study, scientists demonstrated that the Cs2SnI6 is highly redox active. It can be effectively charged/discharged in the presence of iodide redox mediators. Besides, the preparation of a charge regenerator system based on Cs2SnI6 confirmed that charge transfer occurred through the surface state of Cs2SnI6.
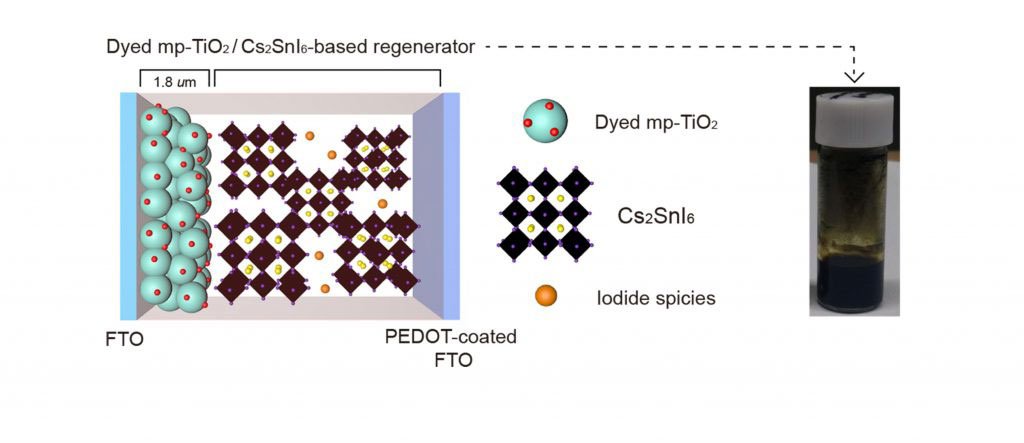
Thus, using Cs2SnI6‐based charge regenerator, scientists designed hybrid solar cells. These hybrid solar cells are expected to generate electric current in the process where the oxidized organic dye returns to its original state.
Byung-Man Kim in the Department of Chemistry at UNIST said, “Due to a high volume of electrical charges in organic dyes that show high connectivity with the surface state of Cs2SnI6, more electric current was generated.”

“Consequently, Cs2SnI6 shows efficient charge transfer with a thermodynamically favorable charge acceptor level, achieving a 79% enhancement in the photocurrent density compared with that of a conventional liquid electrolyte.”
The outcomes of the study demonstrate that the surface state of Cs2SnI6 is the main charge transfer pathway in the presence of a redox mediator and should be considered in future designs of Cs2SnI6‐based devices.
The study is published in the November 2018 issue of Advanced Energy Materials, a highly prestigious journal in materials science.
