Cage structures made with nanoparticles could be a route toward making organized nanostructures with mixed materials, according to researchers at the University of Michigan, who demonstrated how to do so using computer simulations.
The discovery might open the way for photonic materials to manipulate light in ways natural crystals cannot. It also demonstrated an uncommon effect known as entropy compartmentalization, which the team has named.
The structure takes advantage of an unusual physical phenomenon and may allow engineers to manipulate light in novel ways.
Entropy is frequently explained as a disorder in a system. However, it reflects the system’s tendency to maximize its possible states more correctly.
Sharon Glotzer, the Anthony C. Lembke Department Chair of Chemical Engineering, who led the study, said, “We are developing new ways to structure matter across scales, discovering the possibilities and what forces we can use, Entropic forces can stabilize even more complex crystals than we thought.”
While entropy is frequently explained as a disorder in a system, it more correctly reflects its tendency to maximize its possible states. This frequently leads to disorder in the colloquial sense. Oxygen molecules do not huddle together in a corner but spread out to fill a room. However, if you place them in the correct size box, they will spontaneously organize themselves into a recognized structure.
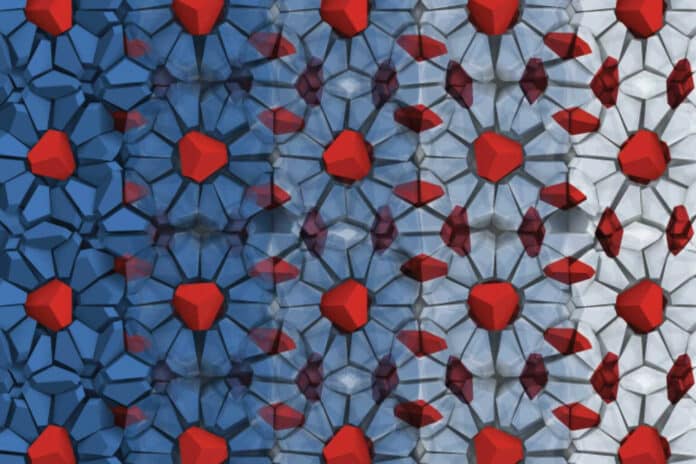
On the left, the blue bipyramid particles fan out around the red bipyramid particles, resembling blue petaled daisies with red centers peeking out of the page.
Red guest bipyramids are nestled between and inside the spheroidal blue-and-gray cages in the stratum of the structure depicted with the cages on the right.
Nanoparticles do the same function. Glotzer’s team previously showed that if bipyramid particles (two short, three-sided pyramids hooked together at their bases) are placed in a sufficiently compact enclosure, they will build structures like fire ice. Fire ice is formed of water molecules that form cages around methane and may both burn and melt.
This substance, abundant beneath the ocean floor, is an example of a clathrate. Clathrate structures are being studied for a variety of uses, including the capture and removal of carbon dioxide from the environment.
Unlike water clathrates, prior nanoparticle clathrate formations lacked gaps that could be filled with other materials to provide new and exciting possibilities for changing the structure’s features. The team wanted to change that.
“This time, we investigated what happens if we change the shape of the particle. We reasoned that if we truncate the particle a little, it would create space in the cage made by the bipyramid particles.” said Sangmin Lee, a recent doctoral graduate in chemical engineering and the paper’s first author.
He took the three middle corners off each bipyramid and found the sweet spot where voids developed in the structure. However, the sides of the pyramids remained intact enough that they didn’t start arranging differently.
When they were the only particle in the system, the voids were filled with more truncated bipyramids. When a second shape was introduced, it became the trapped guest particle.
Glotzer has ideas for creating selectively sticky sides that allow different materials to operate as cage and guest particles. However, there was no glue keeping the bipyramids together in this case. Instead, entropy stabilized the structure.
Glotzer said, “What’s fascinating about the simulations is that the host network is almost completely frozen. The host particles move, but they all move together like a single, rigid object, exactly what happens with water clathrates. But the guest particles are spinning around like crazy—like the system dumped all the entropy into the guest particles.”
Oxygen molecules do not huddle in a corner. However, if placed in the appropriate size box, they will spontaneously organize themselves into a recognized shape. Clathrate structures are being studied for various uses, including capturing and removing carbon dioxide from the environment.
On the other hand, earlier nanoparticle clathrate structures did not have any gaps to fill with different materials that could provide new and fascinating possibilities for changing the structure’s properties.
Sangmin Lee, the paper’s first author and a recent doctoral graduate in chemical engineering, took the three central corners off each bipyramid and discovered the sweet spot where spaces appeared in the structure. However, the sides of the pyramids remained intact enough that they didn’t start organizing differently.
When a second shape was added, that shape became the trapped guest particle. Glotzer has ideas for how to create selectively sticky sides that would enable different materials to act as cage and guest particles. However, in this case, no glue held the bipyramids together.
The cages’ blue bipyramids form a network studded with red guest bipyramids. The blue bipyramids build cages around the red guest particles in a 3D view of the structure.
The essential information in this paragraph is that the host network of water clathrates is nearly frozen, with the host particles moving together like a single, stiff entity while the guest particles spin around like mad.
This system had the most degrees of freedom that the truncated bipyramids could construct in a restricted space. However, the guest particles controlled practically all of it. After the guest particles were gone, the structure dumped bipyramids that had been part of the networked cage structure into the cage interiors.
This study was funded by the Department of Energy and the Office of Naval Research.
Journal Reference:
- Lee, S., Vo, T. & Glotzer,etal. Entropy compartmentalization stabilizes open host–guest colloidal clathrates. Nature Chemistry. DOI: 10.1038/s41557-023-01200-6