A key aspect of biology, such as during photosynthesis, is how molecules change in response to stimuli like light. By combining two, researchers have opened the door for a new age to understand the responses of protein molecules essential for life. Scientists have been striving to untangle the workings of these changes in numerous fields.
The large international research team, led by Professor Jasper van Thor from the Department of Life Sciences at Imperial, has revealed the dance of molecular ‘coherence’ in unprecedented clarity.
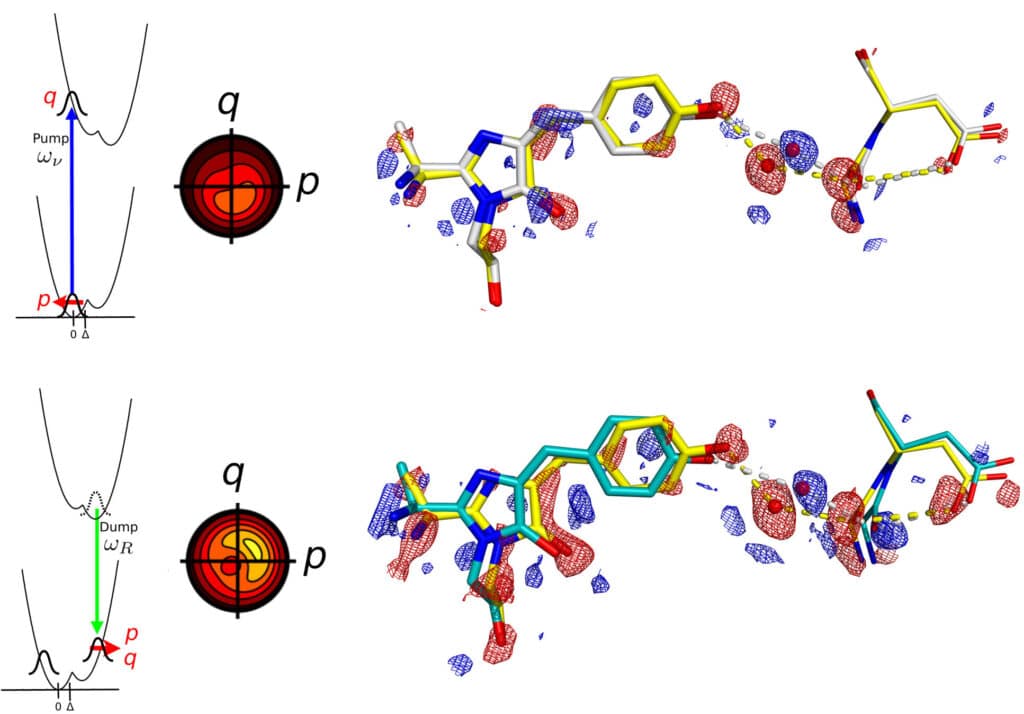
Scientists used a technique called crystallography with spectroscopy technique. The scientists proved that when molecules within the protein they analyzed are optically excited, their first movements are the product of “coherence,” demonstrating the new technique at potent X-ray laser facilities worldwide. Instead of mobility, this demonstrates a vibrational influence on the subsequent biological reaction’s functional component.
This crucial contrast demonstrated experimentally for the first time, demonstrates how the physics of spectroscopy can add fresh perspectives to traditional structural biology crystallographic techniques.
Professor Jasper van Thor from the Department of Life Sciences at Imperial said, “Every process that sustains life is carried out by proteins, but understanding how these complex molecules do their jobs depends on learning the arrangement of their atoms – and how this structure changes – as they react.”
“Using methods from spectroscopy, we can now see ultrafast molecular movements that belong to the so-called coherence process directly in pictorial form by solving their crystal structures. We now have the tools to understand, and even control, molecular dynamics on extremely fast timescales at near-atomic resolution.”
“We hope by sharing the methodological details of this new technique we can encourage researchers in both the fields of time-resolved structural biology and ultrafast laser spectroscopy to explore the crystallographic structures of coherences.”
Previous studies had assumed that all the motions correspond to the biological reaction, meaning its functional motion. But the team found that this was different in their experiments using the new method.
They used “coherent control” to arrive at this result by modifying the laser light to regulate the protein’s movements predictably. Six tests at XFEL sites worldwide were necessary to validate and verify the approach after its initial success in 2018 at Stanford’s LCLS. For each experiment, significant teams and international cooperation were formed.
To apply these theoretical techniques to X-ray crystallographic data rather than spectroscopic data, they integrated the results of these experiments with theoretical techniques adapted from femtochemistry.
They concluded that the ultrafast motions measured with exquisite accuracy on the picometer scale and femtosecond time scale do not belong to the biological reaction but to vibrational coherence in the remaining ground state.
This means that only during the ‘vibrational coherence time,’ the molecules that are ‘left behind’ after the femtosecond laser pulse has passed control the motions that are subsequently observed.
Professor van Thor said: “We concluded that for our experiment, also if coherent control was not included, the conventional time-resolved measurement was dominated by motions from the dark ‘reactant’ ground state, which are unrelated to the biological reactions triggered by the light. Instead, the motions correspond to what is traditionally measured by vibrational spectroscopy and have a very different but equally important significance.
“This was predicted based on previous theoretical work but has been shown experimentally. This will have a significant impact in both the fields of time-resolved structural biology and ultrafast spectroscopy, as we have developed and provided the tools for analysis of ultrafast femtosecond time scale motion.”
Co-author Dr. Sébastien Boutet, from the SLAC National Accelerator Laboratory, which hosts the LCLS, said: “These results represent what is truly unique about the capabilities of x-ray lasers. It demonstrates the type of knowledge on biology in motion that can only be achieved with very short bursts of x-rays and combined with cutting-edge laser technology. We see an exciting future of discovery in this area.”
Co-author Professor Gerrit Groenhof, from the University of Jyväskylä, Finland, said: “Using coherent control to extract the relevant molecular dynamics in the electronic excited state from other motions induced by the excitation laser is essential to understand how photoreceptor proteins have evolved to mediate the photo-activation process. Seeing such a molecular movie of photobiology in action is not only fascinating but may also be the key to unlocking biological principles for designing new light-responsive materials.”
Journal Reference:
- Hutchison, C.D.M., Baxter, J.M., Fitzpatrick, A., et al. Optical control of ultrafast structural dynamics in a fluorescent protein. Nat. Chem. (2023). DOI: 10.1038/s41557-023-01275-1