The liver is an essential organ for turning food into energy in our body. When a person is higher weight, their metabolism can suffer, but we don’t know the exact cause. Researchers from the University of Tokyo compared normal-weight mice with obese ones to learn more.
They found that the way metabolism is controlled in the liver changes between these groups after eating and fasting. In normal mice, metabolism is slowed during eating and speed up during fasting. But in obese mice, it’s the opposite; metabolism speeds up during eating and slows down during fasting.
Obesity affects health by changing how our bodies use energy from food, a process called metabolism. The liver is essential in this process, storing energy and processing food. Researchers studied how obesity affects the liver by comparing normal-weight mice with obese ones after eating and fasting.
They used trans-omics analysis, gathering data on multiple biological processes. Combining this data, they created a network showing how these processes interact.
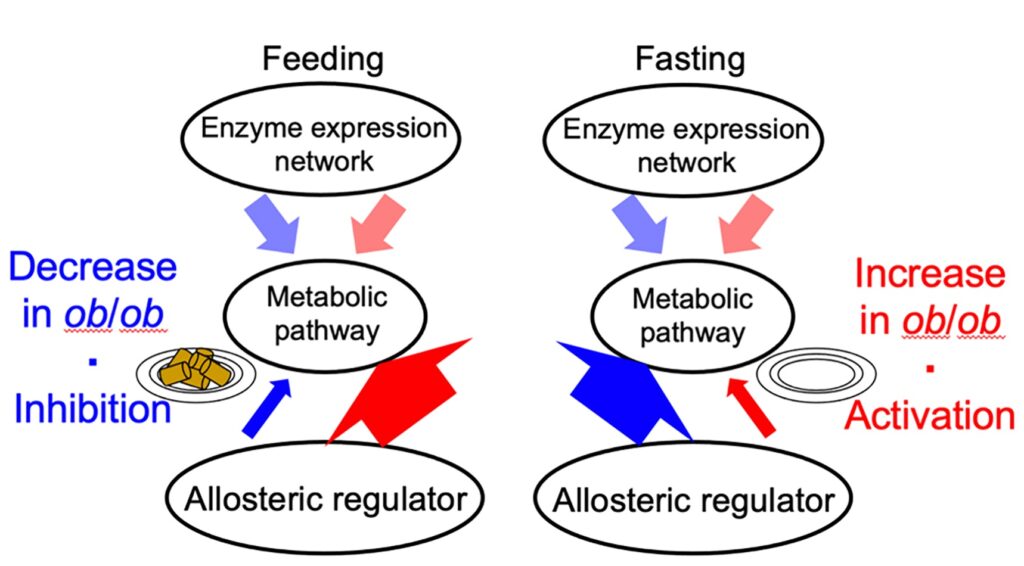
Professor Shinya Kuroda from the University of Tokyo explained “that enzyme and metabolic regulation are suppressed during eating in normal mice. Still, in obese mice, it’s the opposite: these activities increase.”
In obese mice, the balance of energy storage and release in the liver disrupts metabolic stability. This imbalance could lead to fatigue, low energy, and reduced appetite. However, the process that controls cell activity at a genetic level remains relatively stable between eating and fasting, suggesting it’s less influenced by diet.
The researchers believe this disruption in the liver may impact broader metabolic processes in the body. They plan to investigate how metabolic products circulate between the liver and muscles in obese mice, focusing on understanding obesity as a metabolic disease.
The study highlights the importance of obesity on liver function in mice, revealing reversed biological regulation during feeding and fasting states.
Journal reference:
- Yunfan Bai, Keigo Morita, et al., Trans-omic analysis reveals opposite metabolic dysregulation between feeding and fasting in liver associated with obesity. iScience. DOI:10.1016/j.isci.2024.109121.