Enteroendocrine (EE) cells are found throughout the gastrointestinal (GI) tract and represent the most abundant hormone-producing cell type within mammals. EE cells, as a whole, secrete a large variety of hormones, including glucose-dependent insulinotropic polypeptide (GIP, from K cells), cholecystokinin (CCK, from I cells), serotonin (5HT, from enterochromaffin cells), somatostatin (SST, from D cells), peptide YY (PYY, from L cells), and glucagon-like peptide-1 (GLP-1, from L cells), among others.
In response to physiological and nutritional cues, EE cells, through the production of these various hormones, are responsible for regulating multiple aspects of GI activity and nutritional homeostasis. Because of this, EE cells have been implicated in the pathogenesis of GI diseases such as irritable bowel syndrome and inflammatory bowel disease and metabolic diseases such as type 2 diabetes mellitus.
Challenges in converting human intestinal stem cells (ISCs) into functional EE cells, ex vivo, have limited progress in elucidating their role in disease pathogenesis and harnessing their therapeutic potential.
Now, a new technology platform developed at Boston Children’s could set the stage for tapping enteroendocrine (EE) cells to reverse diabetes, obesity, and gastrointestinal conditions like inflammatory bowel disease and irritable bowel syndrome. The platform, described in the journal Nature Communications, is designed to identify drugs that could expand EE numbers, get them to produce more of the needed hormones, or both.
“There’s been interest in exploiting human intestinal stem cells and EE cells to treat disease,” says David Breault, MD, Ph.D., associate chief of the Division of Endocrinology at Boston Children’s. “But the field is still in a nascent stage. This will open new avenues of discovery.”
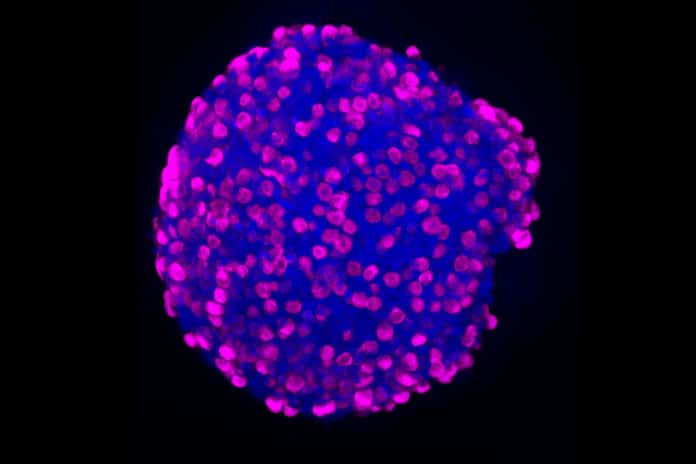
“Similar to other mature intestinal epithelial cells, EE cells are derived from ISCs, which reside within the crypts of Lieberkuhn. In recent years, much progress has been made understanding the mechanisms underlying ISC self-renewal and differentiation using 3D-organoid culture. Using combinations of growth factors and small molecules targeting specific transcriptional regulators and signaling pathways, intestinal organoids can either be maintained predominantly as ISCs or differentiated into mature intestinal cells of either the absorptive or secretory lineages.” Study quotes.
The researchers isolated the intestinal crypts from the patient samples, the “valleys” between intestinal villi where many intestinal stem cells reside. From the intestinal stem cells, they created organoids, three-dimensional mini-organs that replicate the biology of the duodenum and rectum. These locations house relatively large numbers of hormone-producing cells. These organoids then became their platform for systematic testing of libraries of drugs.
“We tried a variety of small molecules with the goal of making more EE cells and/or more hormones,” says Zeve, an attending physician in endocrinology and a member of Breault’s lab.
The system identified three chemicals that, used in different combinations, drove the formation of EE cells and the production of six different hormones: somatostatin, serotonin, glucose-dependent insulinotropic polypeptide (GIP), cholecystokinin, peptide YY, and glucagon-like peptide-1 (GLP-1).
“The ultimate goal would be to identify a medication that induces the secretion of multiple hormones at once,” says Zeve. “This most likely mimics what happens in the body after a meal and may prevent side effects that could occur with the over-production of just one hormone.”
“In summary, we have shown robust differentiation of human EE cells from duodenal and rectal ISCs using Wnt3a-containing differentiation media, and, with respect to enteroids, the addition of the small molecules rimonabant, SP600125, and AS1842856. These protocols improve upon current methods of EE cell differentiation without the use of direct genetic alteration. These studies also provide a platform for future experiments designed to identify endogenous factors regulating EE differentiation, identifying the role and response of EE cells in human GI disease, and further increasing EE cell numbers to potentially be used as personalized cell therapy.” Study quotes.
Journal Reference
- Daniel Zeve, Eric Stas, Joshua de Sousa Casal, Prabhath Mannam, Wanshu Qi, Xiaolei Yin, Sarah Dubois, Manasvi S. Shah, Erin P. Syverson, Sophie Hafner, Jeffrey M. Karp, Diana L. Carlone, Jose Ordovas-Montanes & David T. Breault. Robust differentiation of human enteroendocrine cells from intestinal stem cells; Nature Communications volume 13, Article number: 261 (2022) DOI: 10.1038/s41467-021-27901-5