The fluctuation of photovoltaic and wind power generation is directly related to the weather variability. EPFL scientists are now developing a method that can capture energy source that’s constantly available at river estuaries: osmotic power, also known as blue energy.
Through this research, scientists have shown that using osmosis for power generation could be optimized using light. In the process of osmosis, molecules migrate from a concentrated to a more dilute solution across a semi-permeable membrane in order to balance the concentrations. At river estuaries, electrically charged salt ions move from the salty seawater to the fresh river water. Scientists used this idea to harness this phenomenon to generate power.
To do so, they reproduced the conditions that occur at estuaries and shed light on a system that combines water, salt and a membrane just three atoms thick to generate more electricity. Under the influence of light, the system produces twice as much power as it does in the dark.
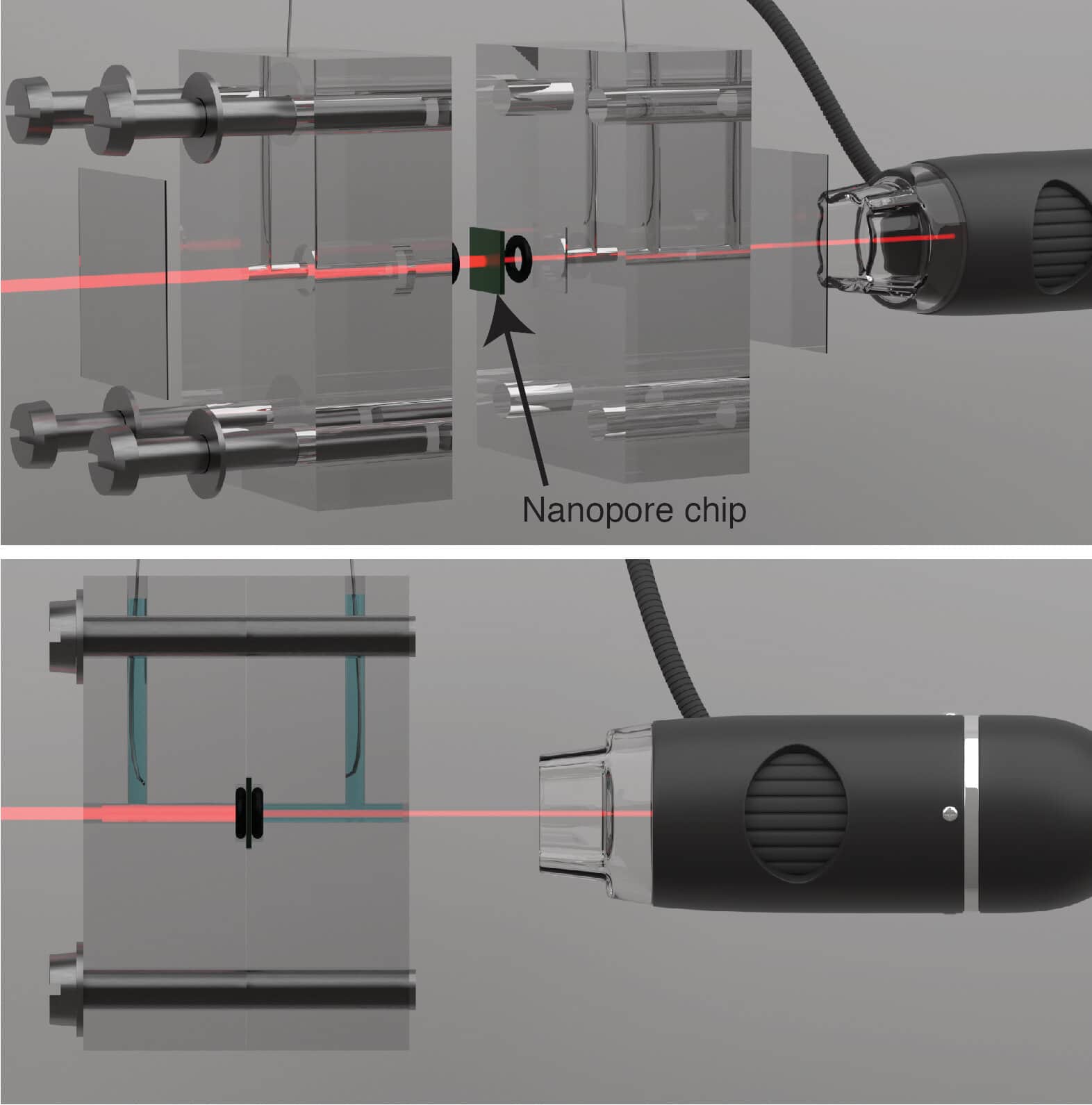
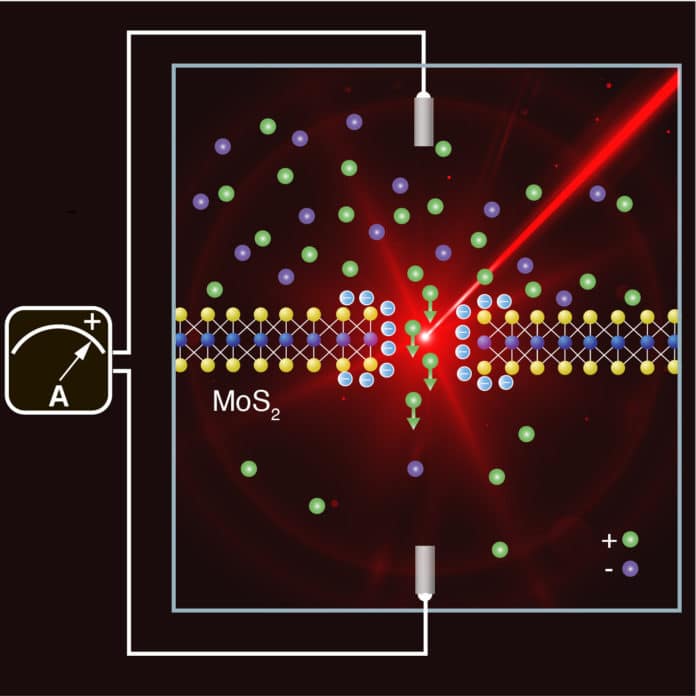
The addition of light means the technology has moved one step closer to real-world application. The system involves two liquid-filled compartments, at markedly different salt concentrations, separated by a molybdenum disulfide (MoS2) membrane. In the middle of the membrane is a nanopore—a tiny hole between three and ten nanometers (one-millionth of a millimeter) in diameter.
Every time a salt ion passes through the hole from the high- to the low-concentration solution, an electron is transferred to an electrode, which generates an electric current.
The system’s power generation potential depends on a number of factors—not least the membrane itself, which needs to be thin in order to generate maximum current. The nanopore also has to be selective to create a potential difference (a voltage) between the two liquids, just like in a conventional battery. The nanopore allows positively charged ions to pass through while pushing away most of the negatively charged ones.
When scientists used low-intensity laser light, they got around two problems at the same time. Releasing light embedded with electrons cLight releases embedded electrons and causes them to accumulate at the membrane’s surface, which increases the surface charge of the material. As a result, the nanopore is more selective and the current flow increases.
Martina Lihter, a researcher at the LBEN said, “Taken together, these two effects mean we don’t have to worry quite so much about the size of the nanopores. That’s good news for large-scale production of the technology since the holes don’t have to be perfect and uniform.”
Michael Graf, the lead author of the paper said, “Essentially, the system could generate osmotic power day and night. The output would double during daylight hours.”
Scientists are now exploring possibilities to scale up production of the membrane, addressing a range of challenges such as optimal pore density. Still, there is a lot of work to do.
This research, led by LBEN, is being conducted as part of a collaboration between two EPFL labs (LANES and LBEN) and researchers at the Department of Electrical and Computer Engineering, University of Illinois Urbana-Champaign.
The study is published in the journal Nature Protocols.