The H+ proton comprises of a single ion of hydrogen, the smallest and lightest of all the chemical elements. These protons commonly occur in water, where a tiny proportion of H2O atoms separate spontaneously. Their amount in a liquid determines whether the arrangement is acidic or basic. Protons are also extremely portable, traveling through water by jumping from one water molecule then onto the next.
How this transport process works in a body of water is undoubtedly known. In any case, the presence of a strong surface can dramatically influence how protons behave, and scientists at present have next to no in the method of devices to gauge these movements at water-solid interfaces.
In this new study, scientists offered the first-ever glimpse of the behavior of protons when water comes into contact with a solid surface, going down to the ultimate scale of a single proton and single charge. They have shown that protons tend to move along the interface between these two mediums.
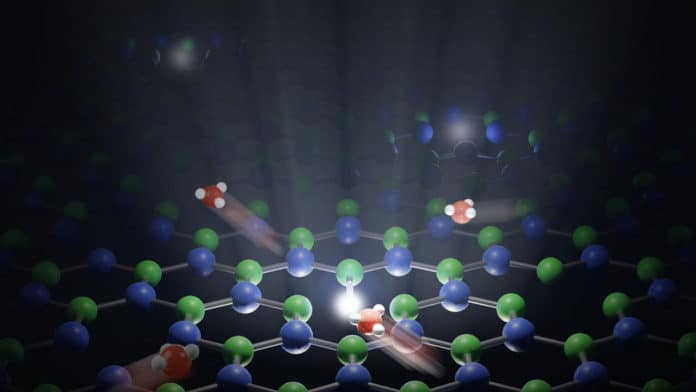
Jean Comtet, a postdoctoral researcher at EPFL’s School of Engineering (STI), said, “We studied the interface between water and a crystal of boron nitride. The surface of the crystal can contain defects.”
“We found that these imperfections act as markers, reemitting light when a proton binds to them. Using a super-resolution microscope, we were able to observe these fluorescence signals and measure the position of the defects within around 10 nanometers – an incredibly high degree of precision. More interesting still, the study revealed new insights in the way crystalline defects are activated.”
“We observed defects on the surface of the crystal lighting up one after another when they came into contact with water. We realized that this lighting pattern was produced by a single proton jumping from defect to defect, generating an identifiable pathway.”
“One of the key findings of the study is that protons tend to move along the water-solid interface. The protons keep on moving, but hugging the surface of the solid.”
Aleksandra Radenovic, professor at EPFL’s Laboratory of Nanoscale Biology (LBEN), adds, “That’s why we see these kinds of patterns. This is a major experimental breakthrough that furthers our understanding of how charges in water interact with solid surfaces.”
Comtet said, “Our observations, in this specific context, can easily be extrapolated to other materials and environments. These discoveries could have important implications in many other fields and disciplines, from understanding biological processes at the cell-membrane interface to designing more efficient filters and batteries.”
Journal Reference:
- Jean Comtet, Direct observation of water-mediated single-proton transport between hBN surface defects. DOI: 10.1038/s41565-020-0695-4