Methane, the largest component of natural gas get wastes 150 billion cubic meters per year during the process called flaring. Thus it leads to almost 400 million tons of CO2 emissions, a significant contributor to global warming.
The gas gets waste in unburned form, thus lead to even greater environmental harm.
The methane is simply a byproduct. It primarily being exploited for the petroleum. Additionally, it is used for producing electrical power and chemicals too. In other words, methane is a vast natural resource, that disposed of through burning.
But now, MIT scientists have found a solution to harness wasted methane. They have developed a new approach that makes it easier to capture wasted methane for use as fuel or a chemical feedstock.
Scientists have found a way to use electricity, which could potentially come from renewable sources, to convert methane into derivatives of methanol, a liquid that can be made into automotive fuel or used as a precursor to a variety of chemical products.
Jillian Dempsey, an assistant professor of chemistry at the University of North Carolina said, “This finding opens the doors for a new paradigm of methane conversion chemistry. It could pave the way to making use of a significant methane supply.”
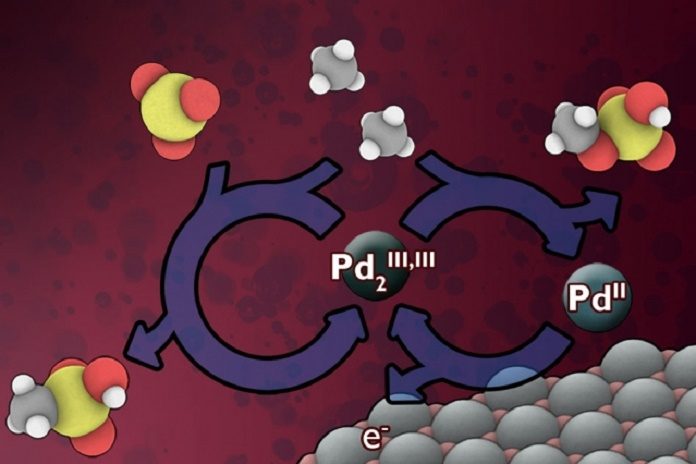
The method is a low-temperature electrochemical process that would continuously replenish a catalyst material that can rapidly carry out the conversion.
MIT chemistry professor Yogesh Surendranath said, “This technology could potentially lead to “a relatively low-cost, on-site addition to existing wellhead operations.”
“The electricity to power such systems could come from wind turbines or solar panels close to the site. This electrochemical process could provide a way to do the methane conversion — a process also known as functionalizing remotely, where a lot of the ‘stranded’ methane reserves are.”
Finding a way to do methane conversion at low cost has been a grand challenge in chemistry for decades. The result of the reaction is a pair of liquid chemicals, methyl bisulfate, and methanesulfonic acid. These can then be processed to make liquid methanol, a valuable chemical intermediate to fuels, plastics, and pharmaceuticals.
Dempsey said, “This work really stands out because it not only reports a new system for selective catalytic functionalization of methane to methanol precursors, but it includes detailed insight into how the system is able to carry out this selective chemistry. The mechanistic information will be instrumental in translating this exciting discovery into an industrial technology.”