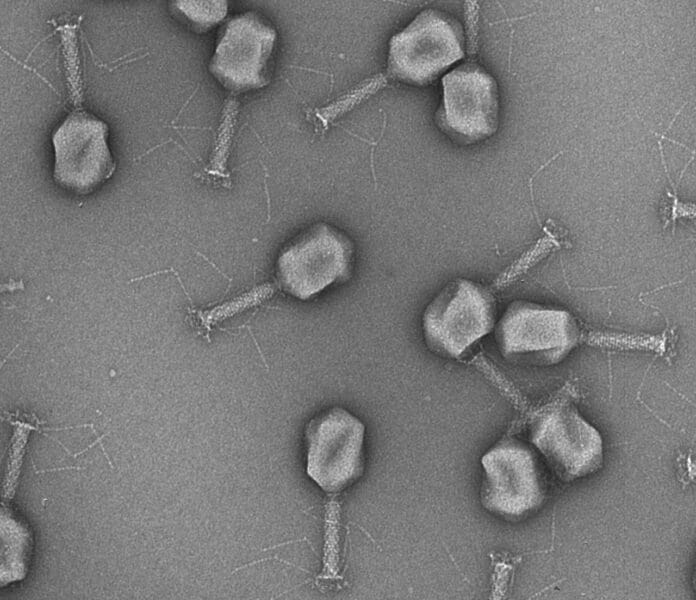
The microorganisms that cause urinary tract infections are growing increasingly resistant to medications. Researchers at ETH Zurich have now devised a quick diagnostic and a new therapeutic strategy based on bacteria-infecting viruses known as phages.
Researchers at ETH Zurich have devised a rapid test and therapy strategy that uses bacteriophages, viruses that attack bacteria, to identify pathogens that cause urinary tract infections rapidly and precisely. This allows for the precise application of an antibiotic. The phages were also genetically changed to make them more effective in killing pathogenic bacteria.
Approximately one in every two women may have cystitis during her lifetime, and many will experience repeated urinary tract infections. Bladder infections are uncomfortable and potentially hazardous, posing a huge challenge for doctors. With antibiotic resistance spreading and developing in urinary tract infections, physicians are frequently obliged to prescribe antibiotics blindly without understanding their efficiency against the bacteria causing the infection. This is because standard tests require many days to detect a specific disease.
ETH Zurich and Balgrist University Hospital researchers have created a quick test using bacteriophages. These virulent viruses naturally prey on bacteria. The phages were also genetically altered by the researchers to increase their effectiveness in eliminating harmful bacteria.
Escherichia coli, Klebsiella, and Enterococci are the three major bacterial strains linked to urinary tract infections, and researchers have discovered efficient phages against each of them. Then, these natural phages were altered so that whenever they recognize and infect bacteria, the result is a simple light signal to monitor. Using this technique, the researchers could accurately identify the pathogenic bacteria from a urine sample in less than four hours.
The technology could allow for the rapid prescription of an appropriate antibiotic following a diagnosis, reducing resistance development and improving antibiotic stewardship. The technology also will enable doctors to forecast which patients are likely to respond particularly well to phage therapy because the strength of the light signal produced in the assay already reflects how effective the phages are at destroying the bacterium.
Phage treatments have been utilized for almost a century. However, with the discovery of penicillin, they went out of favor in Western industrialized countries. They are currently experiencing a rebirth as a result of rising antibiotic resistance.
ETH researcher Samuel Kilcher, one of the study’s two final authors, said, “Phages aren’t interested in completely killing their host, the pathogenic bacterium.”
The scientists altered the phages’ genetic makeup to increase their potency. The treatment is more effective against bacterial strains that have changed portions of their surface so that the phages no longer recognize them thanks to the modified phages, which also manufacture bacteriocins inside the infected host bacterium. The treatment is more successful because of this dual-barreled assault.
Matthew Dunne said, “Many academic and commercial clinical trials are underway worldwide that systematically investigate the potential of natural and genetically optimized phages.”
However, there is still a long way to go before such medicines are routinely used in Western countries. In addition to comprehensive clinical trials, regulatory changes would be beneficial, given that phages are biological entities that co-evolve with their bacterial hosts, i.e., they are always developing.
The current study is a proof of concept, and the researchers will assess the efficacy of the new phage therapy in a clinical trial with selected patients, along with their partners from Balgrist University Hospital.
Journal Reference:
- Du, J., Meile, S., Baggenstos, J. et al. Enhancing bacteriophage therapeutics through in situ production and release of heterologous antimicrobial effectors. Nature Communications. DOI: 10.1038/s41467-023-39612-0
- Meile, S., Du, J., Staubli, S. et al. Engineered reporter phages for detection of Escherichia coli, Enterococcus, and Klebsiella in urine. Nature Communications. DOI: 10.1038/s41467-023-39863-x