Electronic waste (e-waste) is considered the fastest-growing waste stream in the developed world. It comprises a multitude of components with valuable and scarce materials, some containing toxic substances that can contaminate the environment and threaten human health through improper recycling and disposal methods.
Now, a University of Toronto researcher Gisele Azimi has pioneered a method – using captured carbon dioxide to harvest precious metals from electronic waste like batteries and wind turbine materials. Azimi is a University of Toronto professor and the Canada Research Chair for Urban Mining Innovations.
E-waste represents a significant potential source of numerous valuable materials making the recycling of this waste economically fascinating. Recycling electronic waste provides an efficient avenue to gain these vital resources. Researchers say the e-wastes contain about 20 to 38% critical metals, which is significantly higher than the amount of these metals in mined ores – which is only 1 to 2%.
The new process that researchers have developed for recycling e-waste is called supercritical fluid extraction. Previously, the supercritical fluid extraction process was developed for recycling waste electrical and electronic equipment; however, the process mechanism remains unexplored.
The n recycling technique consumes carbon dioxide (CO2) that can be captured directly from the air or from a process that emits carbon dioxide, such as the cement industry and iron making. Researchers use this as a separation technology for extracting metals from electronic waste.
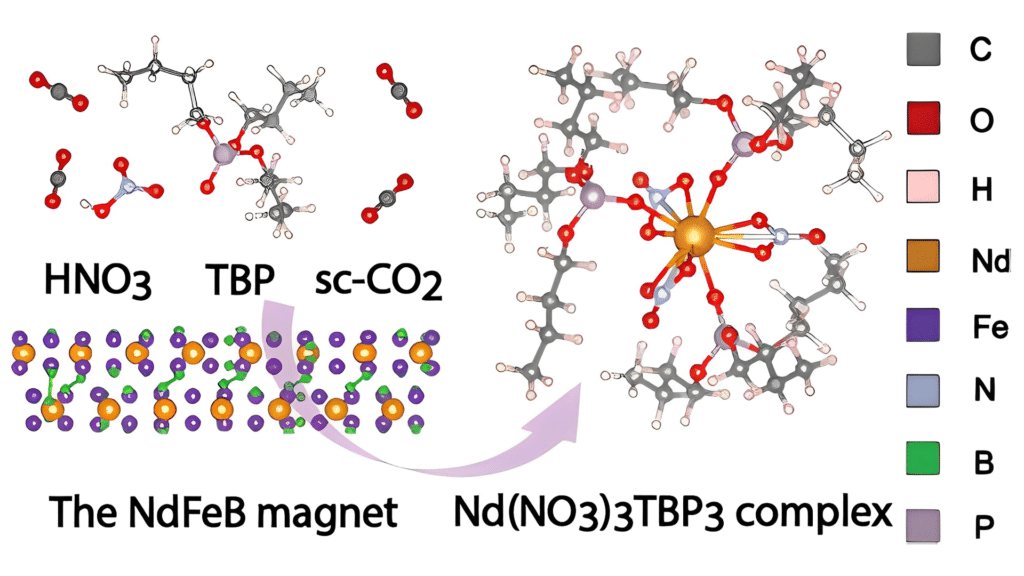
Azimi and her team’s supercritical fluid recycling process involves heating and pressurizing CO2, transforming it into a supercritical fluid. In this state, it can be used to dissolve and extract critical metals from their surroundings. One of the best things about CO2 is that you don’t need to heat it up to high temperatures; only about 30 degrees Celsius is enough to initiate this conversion.
The team approached the Canadian Light Source synchrotron at the University of Saskatchewan to refine and better understand their recycling process. Currently, the method is able to extract metals from car batteries, wind turbine magnets, fluorescent bulbs, and many things in between.
Azimi and her collaborators continue to push the boundaries of their method and carbon-negative recycling. They are working with industry partners on piloting the technique and improving profitability and have set their sights on recovering gold and copper from circuit boards.
Journal reference:
- Jiakai Zhang, Ning Chen, Valeria Morozova, Oleksandr Voznyy, and Gisele Azimi. Investigating Metal–Tributyl Phosphate Complexes during Supercritical Fluid Extraction of the NdFeB Magnet Using Density Functional Theory and X-ray Absorption Spectroscopy. Inorganic Chemistry, 2023; DOI: 10.1021/acs.inorgchem.2c04508
