Organelles are specialized structures that perform the various task inside cells. They have multiple enzymes that work together to execute critical cellular functions.
Researchers at UNIST have mimicked these organelles to create ‘artificial mitochondria.’
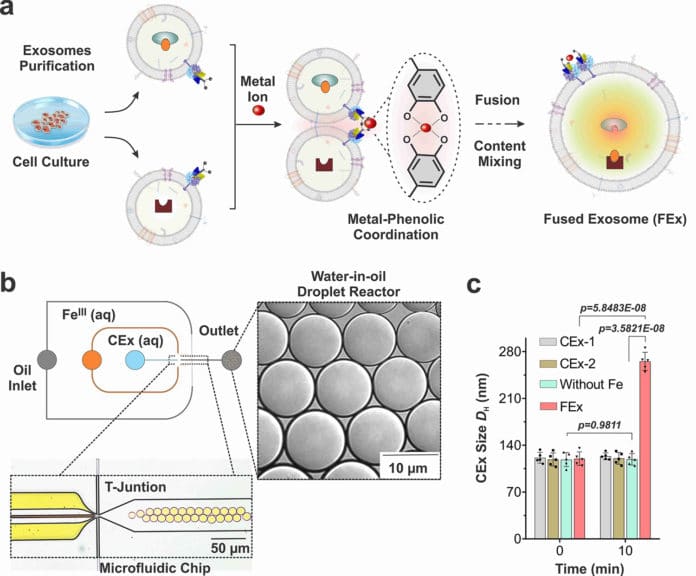
They did so by reprogramming exosomes, a type of extracellular vesicle that contains constituents of the cells that secrete them. During the experiment, researchers used microfluidic droplet reactors to generate droplets of similar size as typical cells.
At first, researchers wanted to facilitate controlled fusion of these exosomes within the droplets while preventing unwanted fusions. They were able to do this by tailoring the exosome surfaces with molecules called catechol. Catechol is a chelating agent that forms complexes with metal ions.
They attached the catechol onto antibodies that target specific cell markers, such as CD9. The complex-forming property of catechol allows them to drive fusions between exosomes when they are mixed with metal ions such as Fe3+. The membrane fusion occurs when the catechols on the surfaces bind to the iron and bring the vesicles to one another.
The system’s effectiveness was determined by loading one type of exosomes with calcein-Co2+ and another with EDTA. When the two vesicles fuse and the contents are mixed, EDTA grabs the Co2+ away from calcein, allowing the latter to fluoresce.
The team realized they successfully detected the fluorescence signal, and the fusion was further confirmed by doubling the measured exosome diameter.
Researchers then loaded the exosomes with different reactants and enzymes. This turned them into biomimetic nano factories. This allows them to produce high-value biomolecules by performing desired biocatalytic transformations in a spatially confined manner, which is impossible using conventional laboratory test tubes.
Scientists demonstrated this multienzyme biocatalytic cascade function by encapsulating glucose oxidase (GOx) and horseradish peroxidase (HRP) inside the exosomes. The GOx first converts glucose into gluconic acid and hydrogen peroxide.
The HRP, in turn, uses the hydrogen peroxide generated in the first reaction to oxidize Amplex Red to a fluorescent product, resorufin. The researchers were even able to take a step further by adding a third enzyme, galactosidase, which converts lactose into glucose into the mix.
Researchers derived the cells from human breast tissues to determine how well these mini reactors can be uptaken and internalized by the cells. They fed the tissues with fused exosome nanoreactors. After that, they observed the internalization over the next 48 hours using various markers and a confocal laser scanning microscope (CLSM).
They found that the cells could uptake these customized exosomes primarily through endocytosis, along with multiple other mechanisms. They further tested the beforementioned GOx-HRP two enzyme system in the cells. It was found that the fused exosomes could successfully manufacture fluorescent products even while being inside the cells.
Researchers were looking forward to creating functional artificial mitochondria capable of producing energy inside the cells based on the findings. They achieved this by reconstituting ATP synthase and bo3 oxidase into earlier exosomes containing GOx and HRP.
As a result, the exosomes fused to create nanoreactors that can produce ATP using glucose and dithiothreitol (DTT). It was found that the fused exosomes could penetrate deep into the core part of a solid spheroid tissue and produce ATP in its hypoxic environment. The activities of these simple organelles were accompanied by marked reduction of pH and reactive oxygen species (ROS) generation. In contrast, free enzymes were unable to penetrate inside these tightly packed spheroids of cells.
Professor Yoon-Kyoung Cho, the corresponding author of the study, said, “Taken together, our results highlight the potential of these exosomes as nanoreactors in regulating the metabolic activity of cells inside spheroids, and in attenuating cell damage due to hypoxia. It is hoped that further research into such artificial organelles will present a new paradigm in various fields such as disease diagnosis and treatment, biotechnology, medicine, and the environment.”
Journal Reference:
- Kumar, S., Karmacharya, M., Michael, I.J. et al. Programmed exosome fusion for energy generation in living cells. Nat Catal (2021). DOI: 10.1038/s41929-021-00669-z