Organic synthesis is making useful chemicals, like medicines, from other chemicals. Chemists usually use source materials to make simple building blocks, like carbonate esters, and put them together to make more complicated structures. The source materials need to react, but often they are toxic, like the common one called phosgene.
Chemists are always searching for new materials that are safer and eco-friendly while still being reactive. They want to use common and safe waste materials, turning them into useful products without making toxic by-products.
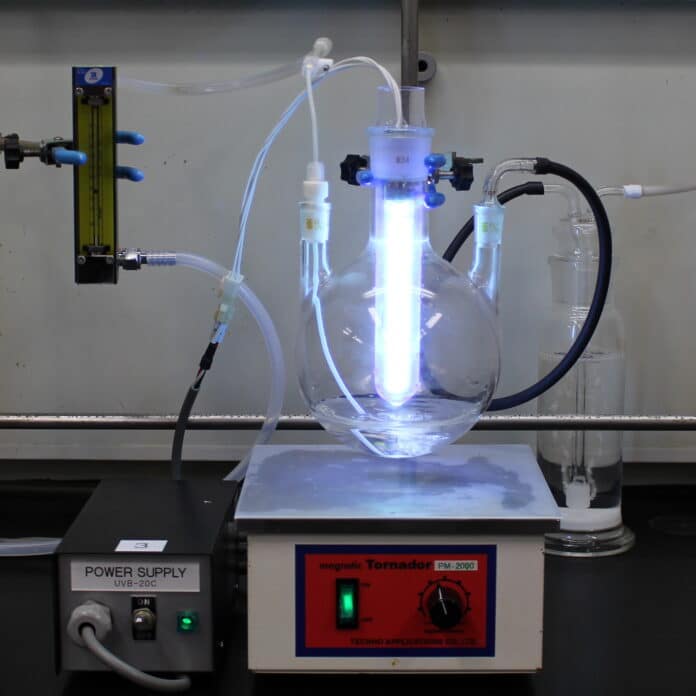
The Kobe University research group of TSUDA Akihiko specializes in the development of one-pot and flow organic synthesis using UV light. The common dry-cleaning and degreasing solvent, perc, can now be transformed into useful chemicals through a new, clean, safe, and affordable method.
This breakthrough, involving on-demand UV activation, might pave the way for recycling perc, promoting a more sustainable and environmentally friendly society.
The research group at Kobe University, led by TSUDA Akihiko, specializes in developing one-pot and flow organic synthesis using UV light. These reactions offer benefits like a closed environment where safe source materials can be photo-activated on demand, eliminating the need for storing potentially toxic materials.
Additionally, any reactive products can be immediately reacted further with other compounds, reducing the risk of environmental leaks. Collaborating with the Japanese material manufacturer AGC Inc., they focused on converting the widely used degreasing and dry-cleaning agent, perc (perchloroethylene), which is both non-harmful and produced globally in large quantities.
In this study, scientists described a method to turn perc into carbonate esters and chloroform, valuable building blocks for further organic synthesis, efficiently and in large quantities without any direct handling of toxic source materials such as phosgene.
Kobe University research group of TSUDA Akihiko said, “Because perc is nonflammable and stable enough to be used as a solvent, its use as a raw material for organic synthesis has received little attention. However, using our original photo-on-demand organic synthesis method, we have succeeded for the first time in simultaneously obtaining industrially important carbonates and chloroforms from that source.”
To minimize the environmental impact of their system, the researchers explored replacing traditional mercury lamps, emitting high-energy UV light, with LED lamps that produce gentler UV light. Despite requiring adjustments to the reaction process, they achieved successful synthesis of the desired products. This adaptation opens up additional possibilities for enhancing the sustainability of organic synthesis processes.
“It is a safe, inexpensive, simple, and environmentally friendly chemical reaction. I expect that this new method of utilizing and upcycling perc, which is used in large quantities around the world, will be a significant step toward the realization of a carbon neutral and sustainable society.”
Journal Reference:
- Ikkei Higashimura, Muge Shele, Toshiki Akamatsu et al. Photo-on-Demand In Situ One-Pot Synthesis of Carbonate Esters from Tetrachloroethylene. The Journal of Organic Chemistry. DOI: 10.1021/acs.joc.3c02588