Unwanted ice accumulation can jeopardize everything from high-altitude aircraft to the operational safety of ships in the Arctic, which is bad news for modern cold climate living. Because ice sticks to most functioning surfaces so tenaciously, removing ice is risky and energy-intensive. As a result, significant efforts have been made in the last few decades to create passive ice-shedding surfaces.
Such studies of ice adhesion have often only been conducted on ice crystallized from pure water. But in all real-world scenarios, freezing water contains dissolved impurities, the results of which still need to be successfully studied in ice adhesion research.
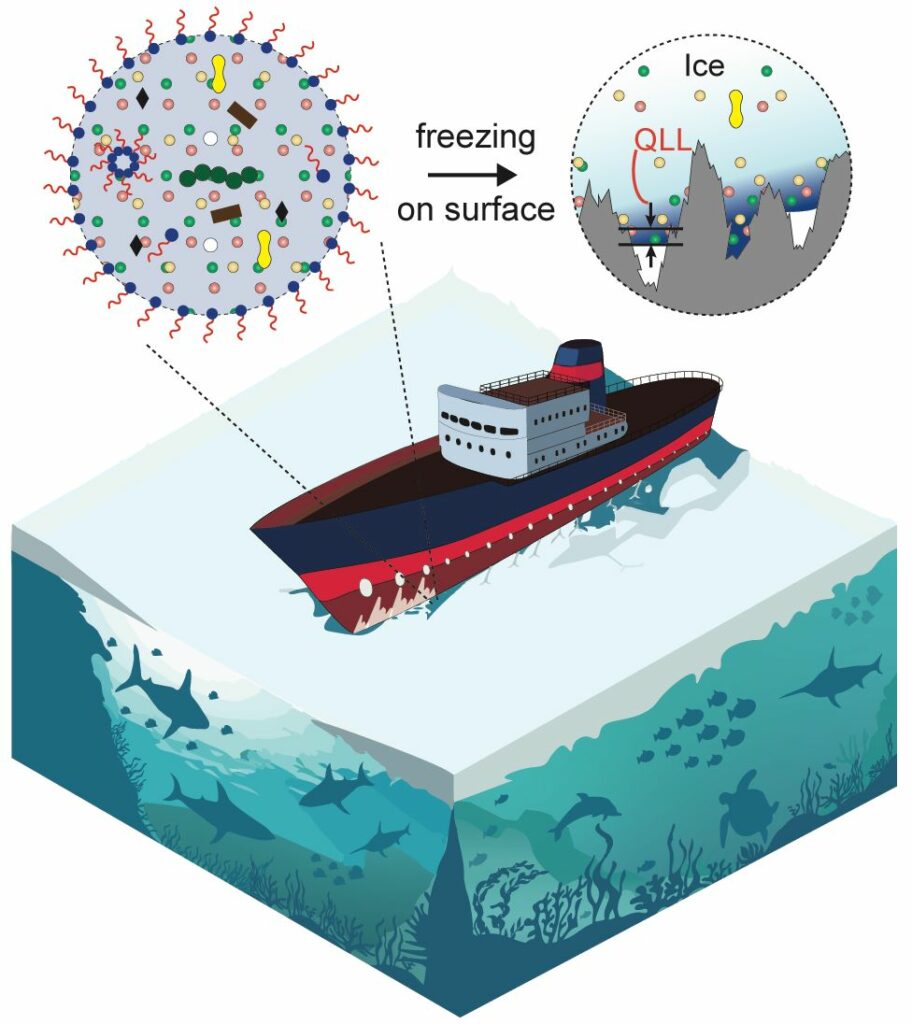
A new study from the University of Illinois Chicago shed light on the fundamental role of various impurities (salt, surfactant, and solvent) commonly found in natural water bodies on ice adhesion on common structural materials.
Senior author Sushant Anand, associate professor of mechanical and industrial engineering at UIC, said, “The natural question that comes to mind is: What is the influence of these compounds on how strongly ice sticks to surfaces?”
In their lab, scientists created ice with varying concentrations of contaminants. They then tested how strongly they clung to different industrial materials. They discovered, somewhat surprisingly, that, under some circumstances, contaminated ice was far less sticky than ice made from pure water.
This slickness was related to how impurity-containing water freezes and the unique structure known as a quasi-liquid layer that forms when ice meets solid matter.
Anand said, “The ice region near a solid has liquid-like properties, and its thickness could contribute to how tightly ice sticks. But this region is challenging to analyze through experiments.”
Scientists studied this layer using molecular dynamics simulations to determine how it changes with different levels of impurities. They discovered that impurities are ejected from the ice by freezing and draining via channels and ice-grain boundaries toward the ice base, where they produce a liquid layer that increases the slipperiness of the ice.
The unexpected test results prompted another query: Why can ships in arctic regions that navigate salt water still struggle with ice formation if low salt concentrations reduce the likelihood of ice adhering to surfaces?
Experiments showed that the rate at which water freezes can affect the migration of contaminants to areas where ice meets a solid. Purer and stronger ice is produced when pollutants are isolated into concentrated pockets or wholly expelled by a long freezing process. Faster freezing maintains the impurities in the ice and their build-up at the ice-solid interface, which results in less adhesion.
Anand said, “Our study represents just the tip of the iceberg, opening new lines of investigation of how impure ice adheres with widespread implications across multiple disciplines.”
Journal Reference:
- Rukmava Chatterjee, Rajith Unnikrishnan Thanjukutty, Chrsitopher Carducci, et al. Adhesion of impure ice on surfaces. Material Horizons. DOI: 10.1039/D3MH01440A
