Promising member of alkali metal-air batteries, potassium air batteries are considered to have more than thrice gravimetric energy density as compared to lithium-ion batteries theoretically. The major issue while designing potassium air batteries was to choose the right electrolyte, the liquid that facilitates the transfer of ions between the anode and cathode. Usually, electrolytes are chosen using a trial-and-error approach based on thumb rules related to several electrolyte properties, followed by exhaustive and time-consuming testing of several electrolyte candidates to achieve the desired performance.
Now, this issue has been resolved by Researchers from Washington University in St. Louis, led by Vijay Ramani, the Roma B. and Raymond H. Wittcoff Distinguished Professor of Environment & Energy at the McKelvey School of Engineering, who have shown how electrolytes for alkali-metal air batteries can be chosen using a single, easy-to-measure parameter.
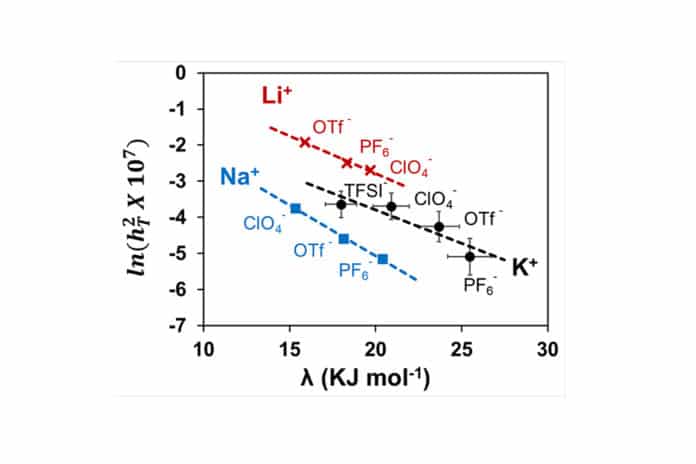
The novel parameter, namely the “Electrochemical” Thiele Modulus, was developed by Ramani’s team after studying the fundamental interaction between salt and solvent in the electrolyte, which influences overall battery performance. This parameter is a measure of the ease of ion transport to and reaction at the end of an electrode surface. This modulus was shown to exponentially decrease with increasing reorganization energy (a measure of the energy needed to modify the solvation sphere of a dissolved species), which could be used to rationally select electrolytes for high-performance metal-air- batteries without trial and error.
This research documents the first time usage of Nobel Prize-winning Marcus Hush theory of electron transfer to study the impact of electrolyte composition on the movement of ions and their reaction at the surface of the electrode.
“We started out trying to better understand the influence of the electrolyte on the oxygen reduction reaction in metal-air battery systems,” said Shrihari Sankarasubramanian, a research scientist on Ramani’s team and lead author of the study. “We ended up showing how the diffusion of ions in the electrolyte and the reaction of these ions on the electrode surface are both correlated to the energy needed to break the solvation shell around the dissolved ions.”
“Showing how a single parameter descriptor of the solvation energy correlates with both ion transport and surface reaction kinetics is a breakthrough advance,” Ramani said. “It will allow us to rationally develop new high-performance electrolytes for metal-air batteries.”
Their work was published July 10 in the Proceedings of the National Academy of Sciences.