Fuel cells are an eco-friendly energy source that obtains energy through the reverse reaction of water electrolysis.
The catalyst that enhances the reaction efficiency is directly connected to the performance of the fuel cell. A catalyst is a substance that enhances chemical reactions. One such catalyst for fuel cells- PBMO (Pr0.5Ba0.5MnO3-δ)- operates even when directly used as a hydrocarbon, not hydrogen, and offers high ionic conductivity.
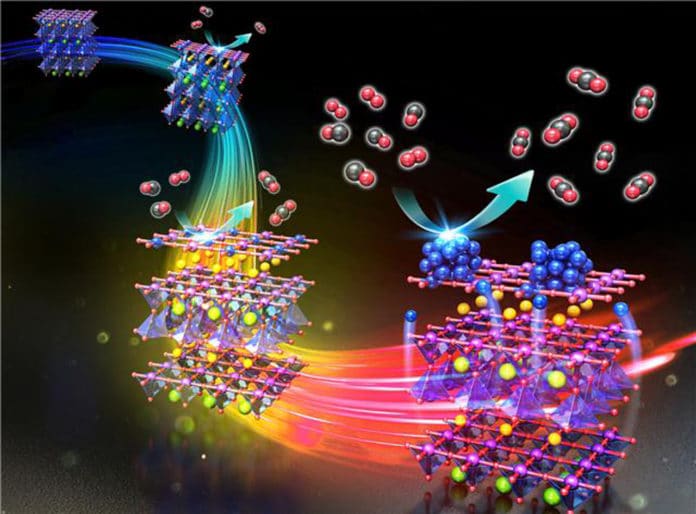
To this, a POSTECH-UNIST joint research team has taken a step closer to developing high-performance catalysts by uncovering the ex-solution and phase transition phenomena at the atomic level for the first time. A joint research team of Professor Jeong Woo Han and Ph.D. candidate Kyeounghak Kim from the Department of Chemical Engineering at POSTECH and Professor Guntae Kim from UNIST has uncovered the mechanism which PBMO – a catalyst used in fuel cells.
The PMBO is transformed from perovskite structure to layered structure with nanoparticles ex-solution* to the surface, confirming its potential as an electrode and a chemical catalyst. It exhibits high ionic conductivity as it changes to a layered structure under a reduction environment that loses oxygen. Simultaneously, the ex-solution phenomenon occurs in which the elements inside the metal oxide segregate to the surface.
This phenomenon occurs mainly in a reduction environment without any particular process when elements in the material rise to the surface, the fuel cell’s stability, and performance improve immensely.
However, designing these materials is quite difficult as the process behind this was unknown.
Scientists focused on the features and confirmed that the process goes through a phase transition, particle ex-solution, and catalyst formation. To prove this, they used the first-principles calculation based on quantum mechanics and the in-situ XRD** experiment that allows the observation of real-time crystal structural changes in materials. Scientists also confirmed that the oxidation catalyst developed this way displays up to four times better performance than the conventional catalysts, verifying that this study applies to various chemical catalysts.
Professor Jeong Woo Han, who led the study, said, “We were able to accurately understand the materials in atomic units that were difficult to confirm in previous experiments, and successfully demonstrated it thus overcoming the limitations of existing research by accurately understanding materials in atomic units, which were difficult to confirm in existing experiments, and successfully demonstrating them. Since these support materials and nanocatalysts can be used for exhaust gas reduction, sensors, fuel cells, chemical catalysts, etc., active research in numerous fields is anticipated in the future.”
Journal Reference:
- Kyeounghak Kim et al. Mechanistic insights into the phase transition and metal ex-solution phenomena of Pr0.5Ba0.5Mn0.85Co0.15O3−δ from simple to layered perovskite under reducing conditions and enhanced catalytic activity†. DOI: 10.1039/D0EE02875D