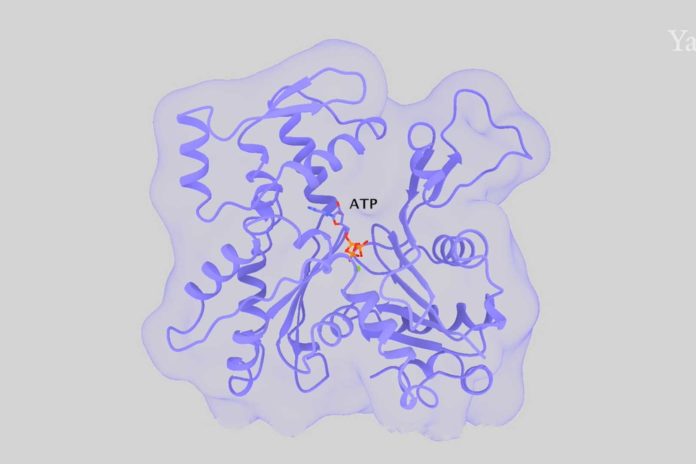
Actin is the most abundant protein in most eukaryotic cells. It is highly conserved and participates in more protein-protein interactions than any known protein. Actin filaments comprise a major part of the cytoskeleton of eukaryotic cells and serve as tracks for myosin motor proteins. The filaments assemble from actin monomers with a bound ATP.
Understanding how actin filaments assemble, disassemble, and interact with numerous regulatory proteins depends on knowing the structure of the filament. In a new study by Yale University, scientists determined high-quality structures of actin filaments with bound AMP-PNP (a slowly hydrolyzed ATP analog), ADP and phosphate, or ADP by cryo-electron microscopy.
For the study, scientists used advanced cryo-microscopy to determine the highest resolution structures of actin filaments, which answered these and other questions.
Tom Pollard, a Yale University scientist, said, “We understood the chemistry, but until now we could not see how the processes work at the atomic level.”
The study is published in the journal Proceedings of the National Academy of Sciences.