Rice University scientists used a dash of salt to simplify the creation of two-dimensional materials that combined transition metals and chalcogens. Scientists believe that the discovery could lead to smaller and faster transistors, photovoltaics, sensors, and catalysts.
Boris Yakobson, a Rice professor of materials science and nanoengineering and of chemistry, was the go-to expert when a group of labs in Singapore, China, Japan and Taiwan primarily determined that salt reduces the temperature at which some elements interact in a chemical vapor deposition (CVD) furnace. Thus, it is easy to frame molecule-thick layers like graphene with the capability to redo their substance organization for particular layer-material and in like manner electrical, optical, synergist and other helpful properties.
Scientists experimented their technique with CVD to create 47 compounds of metal chalcogenides. Most of the new compounds had two ingredients, but some were alloys of three, four and even five. A significant number of the materials had been envisioned and even pined for, but never made.
In the CVD procedure, atoms energized by temperatures — for this situation in the vicinity of 600 and 850 degrees Celsius (1,112 and 1,562 degrees Fahrenheit) — shape a gas and at last settle on a substrate, connecting to molecules of reciprocal science to frame monolayer precious stones.
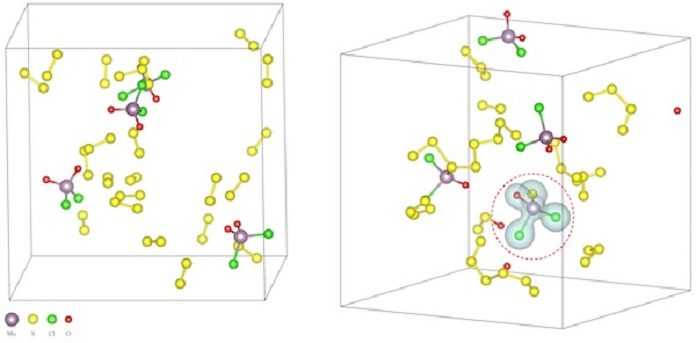
Researchers already suspected salt could facilitate the process. They also analyzed the molecular model to learn why salt made it easier to melt metals with chalcogens and get them to react. That would help them learn if it might work within the broader palette of the periodic table.
Yakobson said, “Whether in the form of common table salt (sodium chloride) or more exotic compounds like potassium iodide, salt was found to allow chemical reactions by lowering the energetic barrier that otherwise prevents molecules from interacting at anything less than ultrahigh temperatures.”
“I call it a ‘salt assault’. This is important for synthesis. First, when you try to combine solid particles, no matter how small they are, they still have limited contact with each other. But if you melt them, with salt’s help, you get a lot of contact on the molecular level.”
“Second, salt reduces the sublimation point, where a solid undergoes a phase transformation to gas. It means more of the material’s component molecules jump into the gas phase. That’s good for general transport and contact issues and helps the reaction overall.”
Scientists found that the procedure doesn’t encourage the development of the 2-D-material itself specifically to such an extent as it takes into account the arrangement of the middle of the road oxychlorides. These oxychlorides at that point prompt the 2-D chalcogenide development.
Yakobson said, “Detailing this process required intensive atom-by-atom simulations. These took weeks of heavy-duty computations of the quantum interactions among as few as about 100 atoms – all to show just 10 picoseconds of a reaction. We only did four of the compounds because they were so computationally expensive, and the emerging picture was clear enough.”
Co-authors of the paper are Jiadong Zhou, Fucai Liu, Qundong Fu, Qingsheng Zeng, Hong Wang, Yu Chen, Juan Xia, Ting Yu and Zexiang Shen of Nanyang Technological University, Singapore; Junhao Lin and Kazu Suenaga of the National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan; Xiangwei Huang, Guangtong Liu, Yao Zhou and Qian Liu of the Chinese Academy of Sciences, Beijing; Huimei Yu of East China University of Science and Technology, Shanghai; Di Wu and Chuang-Han Hsu of the National University of Singapore; Changli Yang and Li Lu of the Chinese Academy of Sciences and Collaborative Innovation Center of Quantum Matter, Beijing; and Hsin Lin of the National University of Singapore and the Institute of Physics, Academia Sinica, Taipei, Taiwan.
Scientists have reported the results this week in Nature.