The lithium-ion battery is workhorse power source in many applications. But how do its charging and discharging cycles happen, is still remains a mystery?
In a new study by the U.S. Department of Energy’s (DOE) Argonne National Laboratory, scientists made a breakthrough by discovering a microscopically thin layer that forms at the interface between the liquid electrolyte and solid electrode. Scientists referred this layer as “solid-electrolyte interphase” or SEI.
All good lithium-ion batteries have well-functioning SEIs. It prevents detrimental reactions from occurring at the interface, while at the same time allowing the important lithium ions free rein to move between the electrolyte and electrode.
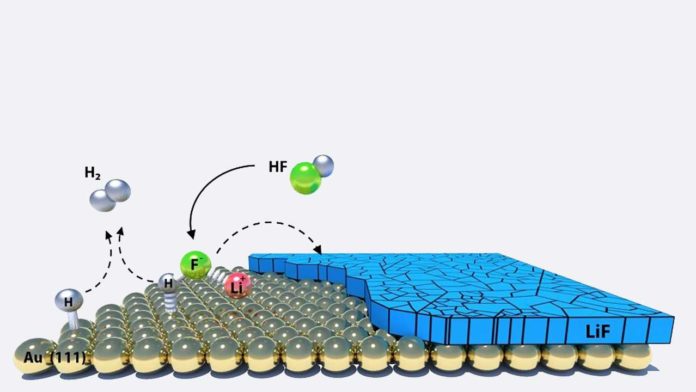
Scientists deciphered the chemistry behind one of the more common components of the SEI in typical lithium-ion batteries, lithium fluoride. Based on both experimental and computational results, their findings showed that this phase forms in battery charge by the electrochemical reaction of hydrogen fluoride, producing hydrogen gas and solid lithium fluoride.
This reaction depends highly on the electrode material, which could be a metal, graphene or graphitic material, and thus demonstrates the importance of catalysis in battery operation.
Dusan Strmcnik — a co-principal investigator and assistant chemical engineer in the Materials Science Division (MSD) said, “Battery performance is highly dependent on the quality of the SEI. If the chemistry and the role of individual components of the SEI are understood, the SEI could be tuned to improve battery performance.”
“More importantly, such understanding would significantly improve our predictive ability of battery lifetime, which is of extremely high value to an electric car manufacturer.”
Scientists discovered a new way to monitor the concentration of the hydrogen fluoride, a highly detrimental impurity that forms from a reaction between trace amounts of moisture and the salt (LiPF6) in the electrolyte. This monitoring capability should prove vital to future basic science studies of the SEI.
To Argonne Distinguished Fellow and co-principal investigator Nenad Markovic, the study’s results are already having a commercial impact. “Our findings are already being implemented in lithium-ion cells at the Battery Cell Competence Center of the BMW Group. They will also open new opportunities for the improvement of existing, and the design of new, lithium-ion technologies.”
The study is published in the journal Nature Catalysis.