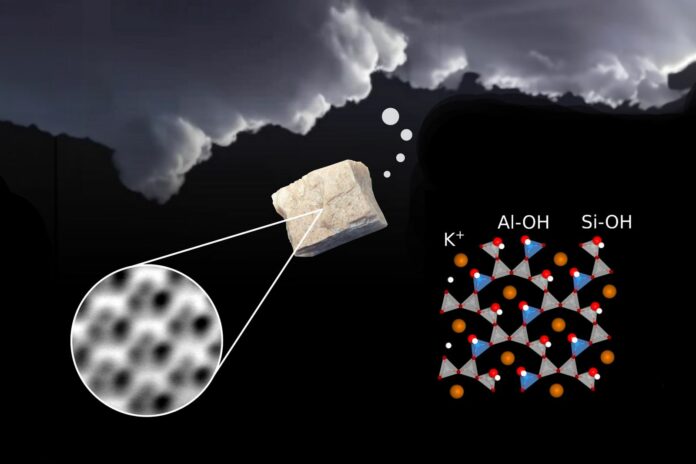
Microcline feldspar is a type of mineral found on Earth and plays a significant role in keeping it balanced. It helps store carbon, move potassium and water around, and make soil and ice in the atmosphere. To get how this all works, we need to figure out what the surface of the microcline looks like at the tiniest level and how it interacts with water, which is everywhere.
Scientists are trying to understand why Feldspar is so good at holding onto water and helping clouds form. But, using a highly sensitive atomic force microscope, researchers at TU Wien have discovered that the unique shape of the feldspar surface is just right for grabbing onto the OH groups of hydrogen and oxygen, which are the building blocks of water. This makes feldspar an excellent anchor for water molecules.
This microscope scans the crystal’s surface with a tiny tip, going point by point. The force between the end and the surface creates a highly detailed image, allowing scientists to determine each atom’s position precisely.
Giada Franceschi, the study’s first author, said, “We placed a piece of feldspar in the microscope’s vacuum chamber and split it in half to obtain a pristine and clean surface. We were puzzled by the results: The images of the surface looked different from what common theories had predicted.”
The reason was soon discovered: Small pockets of water trapped inside the stone were responsible. A small amount of water vapor is released when the stone is broken. This vapor sticks to the newly exposed surface, and the water molecules split, creating hydroxyl (OH) groups.
Giada Franceschi said, “Under the microscope, you don’t see the feldspar surface itself but a surface covered with hydroxyl groups. In nature, the feldspar surface is also covered with such a hydroxyl layer.”
Because of the specific shape of the feldspar crystal, these hydroxyl groups are arranged in a manner that makes them perfect points for attaching water molecules. It’s like having building blocks that fit together perfectly. The hydroxyl layer acts as the ideal link between the feldspar and the water, especially when the water turns into ice and attaches to the crystal.
Ulrike Diebold said, “The bond is established very easily and quickly, and it is also very stable. To remove the hydroxyl layer from feldspar, one would have to heat it to high temperature.” Computer simulations also support this finding.”
“The results provide insight into why specific crystals in our atmosphere are particularly well-suited as cloud-forming nucleation seeds. Especially in the face of climate change, it is important to understand the physics of cloud formation better. And sometimes, as the research project at TU Wien shows, one has to delve deep into the world of atoms.”
Journal Reference:
- G. Franceschi et al., How Water Binds to Microcline Feldspar (001), J. Phys. Chem. Lett. 2024, 15, 15–22. DOI: 10.1021/acs.jpclett.3c03235