Articular cartilage is the smooth, white tissue that covers the ends of bones where they come together to form joints. Healthy cartilage in our joints makes it easier to move. It allows the bones to glide over each other with very little friction.
Articular cartilage can be damaged by injury or normal wear and tear. Once injured, it can not regrow or heal on their own. Now, scientists at the UNIST, have identified the mechanisms of how frog embryos develop to form facial cartilages. They also have identified a novel potential therapeutic target that could pave the way for regenerative treatments for arthritis in humans.
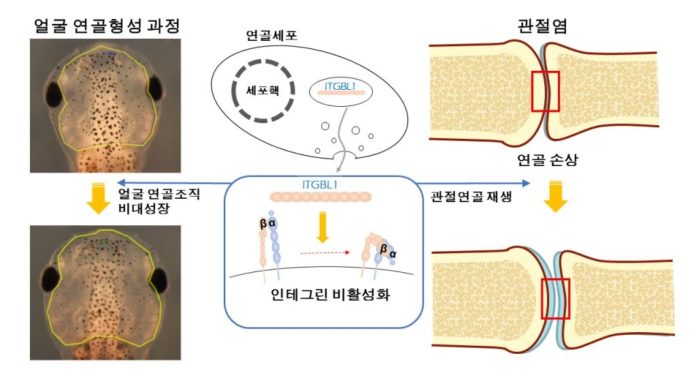
Pharyngeal arches of vertebrate embryos develop to form various facial tissues including cartilage, bone, muscle, and other connective tissues. The pharyngeal arches of the Xenopus embryo provide accessible and tractable tissues to study the interplay among various tissues during cartilage formation. Through the study of Xenopus Laevis, the research team has identified, for the first time, integrin-β–like 1 (ITGBL1), a secreted protein involved in cartilage formation, plays a major role in the protection against osteoarthritis (OA) development in joint cartilage.
Joint destruction in both rheumatoid arthritis and osteoarthritis occur when the soft bone marrow, that is, the cartilage that cushions the joints wears away. Unlike other self-repairing tissues, cartilage has a low regenerative capacity. It lacks vascular, neural, and lymphatic networks, as well as various local progenitor cells. For these reasons, it is challenging to restore full tissue function in damaged or diseased articular cartilage.
To examine whether ITGBL1 is involved in cartilage development, the team knocked down Itgbl1 expression using a splice-blocking morpholino oligo (Itgbl1-MO) in Xenopus laevis embryos. They found that developing chondrocytes express integrin-β–like 1 (Itgbl1) at specific stages, inhibiting integrin signaling and promoting chondrogenesis.
Unlike cytosolic integrin inhibitors, ITGBL1 is secreted and physically interacts with integrins to down-regulate activity. We observed that Itgbl1 expression was strongly reduced in the damaged articular cartilage of patients with osteoarthritis (OA).
Ectopic expression of Itgbl1 protected joint cartilage against OA development in the destabilization of the medial meniscus–induced OA mouse model. Their results reveal ITGBL1 signaling as an underlying mechanism of protection against destructive cartilage disorders and suggest the potential therapeutic utility of targeting ITGBL1 to modulate integrin signaling in human disease.
Professor Park said, “The unique function of ITGBL1 as a secreted integrin inhibitor points toward new approaches to treat integrin-mediated human diseases and destructive cartilage disorders.”
The findings of this research have been published in the online edition of Science Translational Medicine, the sister journal of Science on October 10, 2018.