The proliferation of plastic products in the last several decades has been extraordinary. Quite simply, humans are addicted to this nearly indestructible material. Over 300 million tons of plastic produced every year, 50% of which is for single-use purposes – utilized for just a few moments, but on the planet for at least several hundred years.
Even if producing tonnes of plastic, the details of what goes on at the atomic scale during the plastics production process are still unclear.
Now, scientists at the DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab) along with the Eindhoven University of Technology in the Netherlands have developed a new technique that offers an atomic resolution of magnesium chloride, a material involved in the production of the most common plastic.
Scientists could use electron beams directly, but doing this damages the delicate material, thus they used pulsed electron beams in an electron microscope and generated the first of its kind images of magnesium chloride.
Christian Kisielowski, lead author of the study and staff scientist at Berkeley Lab’s Molecular Foundry, a nanoscale science user facility said, “If you had asked me 10 years ago if we could use pulsed electron beams to image beam-sensitive materials with atomic resolution, I would not have believed it. Now it is possible, and it has allowed us to study important material for the plastics industry.”
“This is a game changer for imaging a wide range of materials that are normally damaged inside an electron microscope. Besides magnesium chloride, for example, pulsed electron beams could also be used to study soft membranes and plastics in general.”
Magnesium chloride acts as a support structure for catalysts used to make plastics. But, its exact function remains a mystery.
Scientists noted, “Atomic-scale images of magnesium chloride would help clarify its role in plastics production and could help pave the way to more specialized and sustainable plastics.”
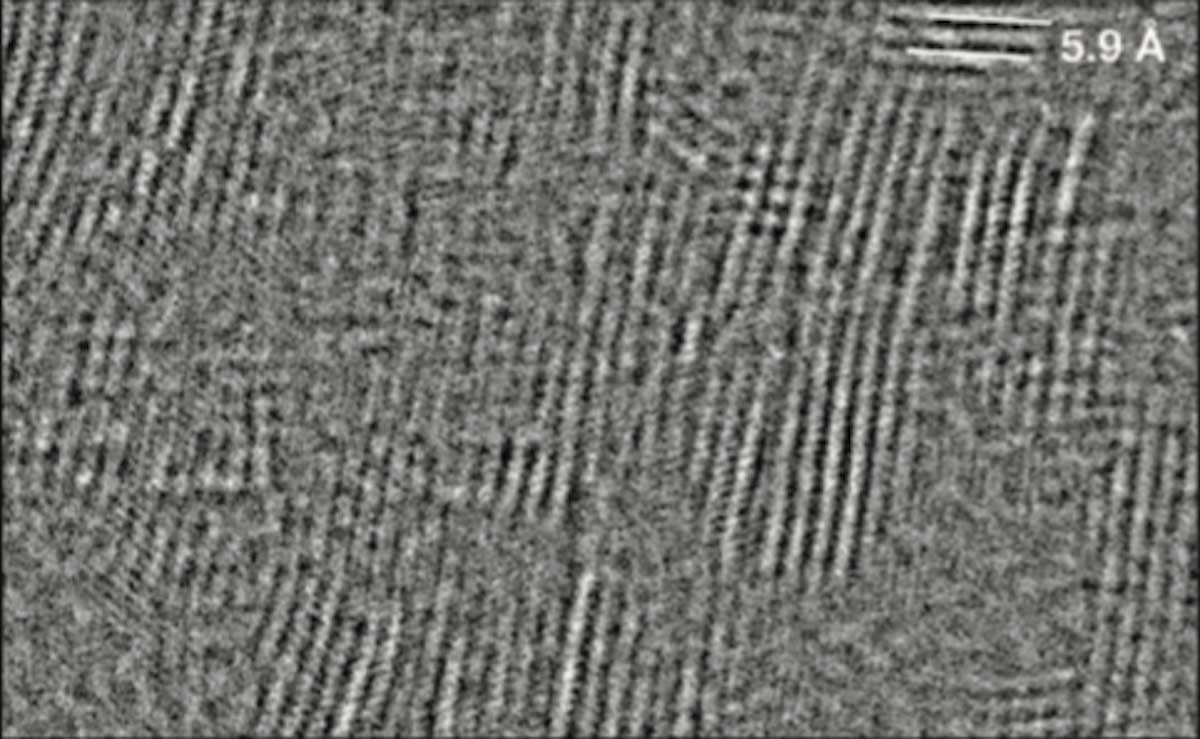
In previous attempts, scientists failed to image the material as it is presented in two types of crystal structures with slightly different arrangements of atoms. Electron beams itself affects the material structure, making it difficult to interpret which structure is being imaged.
Here’s when scientists collaborated with Eindhoven University of Technology and Dow to develop a technique that offers periodic pulses of electrons instead of a continuous electron beam for imaging the material. Using a modified electron microscope at Eindhoven, the researchers found that by pulsing the electron beam like an extremely fast strobe light with one pulse every 160 picoseconds (1 picosecond is one trillionth of a second), the material can essentially “heal” itself between pulses.
Scientists also understood that samples get damaged in an electron microscope when atoms are knocked out of position or molecules are split into smaller particles. They also learned that the accumulation of atomic vibrations caused by the electron beam is equally important.
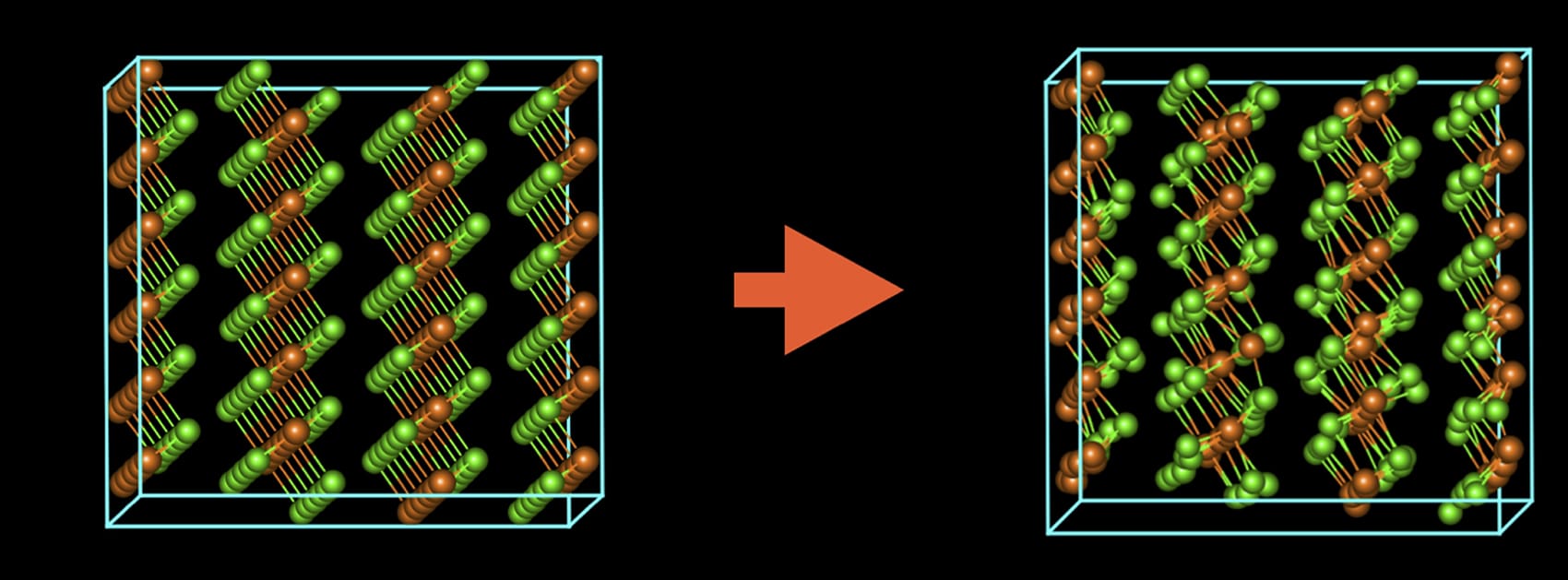
By pulsing the beam in time with these vibrations, the researchers preserved the material’s original atomic structure and revealed that sheets of magnesium chloride stack on top of each other in an irregular arrangement like a haphazard stack of books, which distinguishes it from other materials.
Another problem that other researchers have grappled with when imaging magnesium chloride is that when the material is exposed to air, it changes in both chemical content and crystal structure (the way its atoms are arranged in space). But when using conventional electron microscopy techniques, the sample is exposed to air as it is transferred to the microscope.
Kisielowski said, “We were able to minimize the material’s exposure to air prior to putting it inside the microscope by using a special vacuum-sealed holder. We also learned how to handle air-sensitive materials, and that was a key element of this whole thing.”
Scientists are now planning to study the relationships between these structures and the properties of plastics, paving the way toward more specialized and sustainable plastics.
Their findings were reported in Advanced Functional Materials.
