Developing efficient and stable electrocatalysts is crucial for the electrochemical production of pure and clean hydrogen. For practical applications, an economical and facile method of producing catalysts for the hydrogen evolution reaction (HER) is essential.
Scientists at the UNIST have recently devised a new water-splitting hydrogen catalyst. Scientists also demonstrated catalytic performance of the device and found that it is superior in many ways to the commercial Pt/C catalysts.
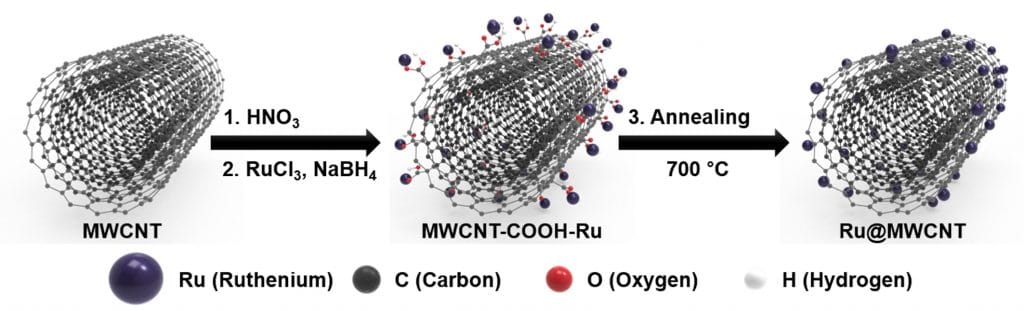
The catalyst contains ruthenium (Ru) nanoparticles uniformly distributed and anchored on the surface of multiwalled carbon nanotubes (MWCNTs), or (Ru@MWCNT). Scientists denoted that the catalyst is simple to synthesize and can be mass-produced.
Professor Jong-Beom Baek at UNIST said, “In addition to introducing highly-efficient and stable catalysts that surpass the characteristics of existing materials, this study aims at evaluating the catalytic performance of catalyst electrodes, which is an essential part of commercialization.”
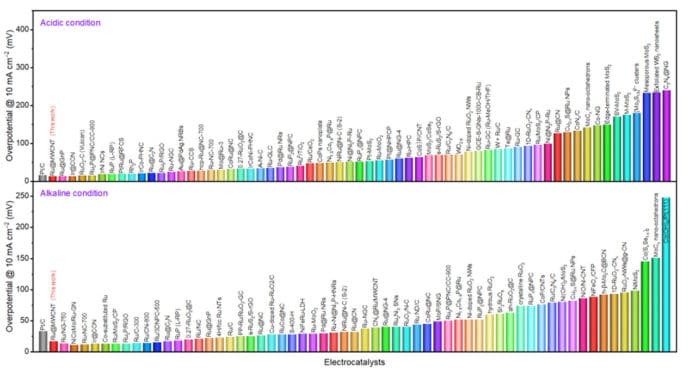
“The Ru@MWCNT catalyst exhibits superior electrochemical properties over the previously announced metal-organic catalysts. The catalyst demonstrates excellent HER performance with low overpotentials (See Figure 1), outstanding durability, and high turnover frequencies in both acidic and alkaline conditions.”
The Ru@MWCNT catalysts take the structure, in which ruthenium (Ru) nanoparticles are uniformly distributed and anchored on the surface of multiwalled carbon nanotubes (MWCNTs). Due to the smaller particle size distribution and particle uniformity, it displays excellent HER performance, and for this, a manufacturing process has also been developed.
Do Hyung Kweon (Combined M.S/Ph.D. of Energy and Chemical Engineering, UNIST), the first author of the study said, “The existing method of combining Ru and CNTs, there is a tendency for Ru particles stick together and by steadily increasing the size of the agglomerate during the heat treatment. We suppress this particle agglomeration via the introduction of ‘Ru salt’ and ‘-COOH,’ and this enabled the uniform distribution of Ru nanoparticles on the surface of the MWCNT.”
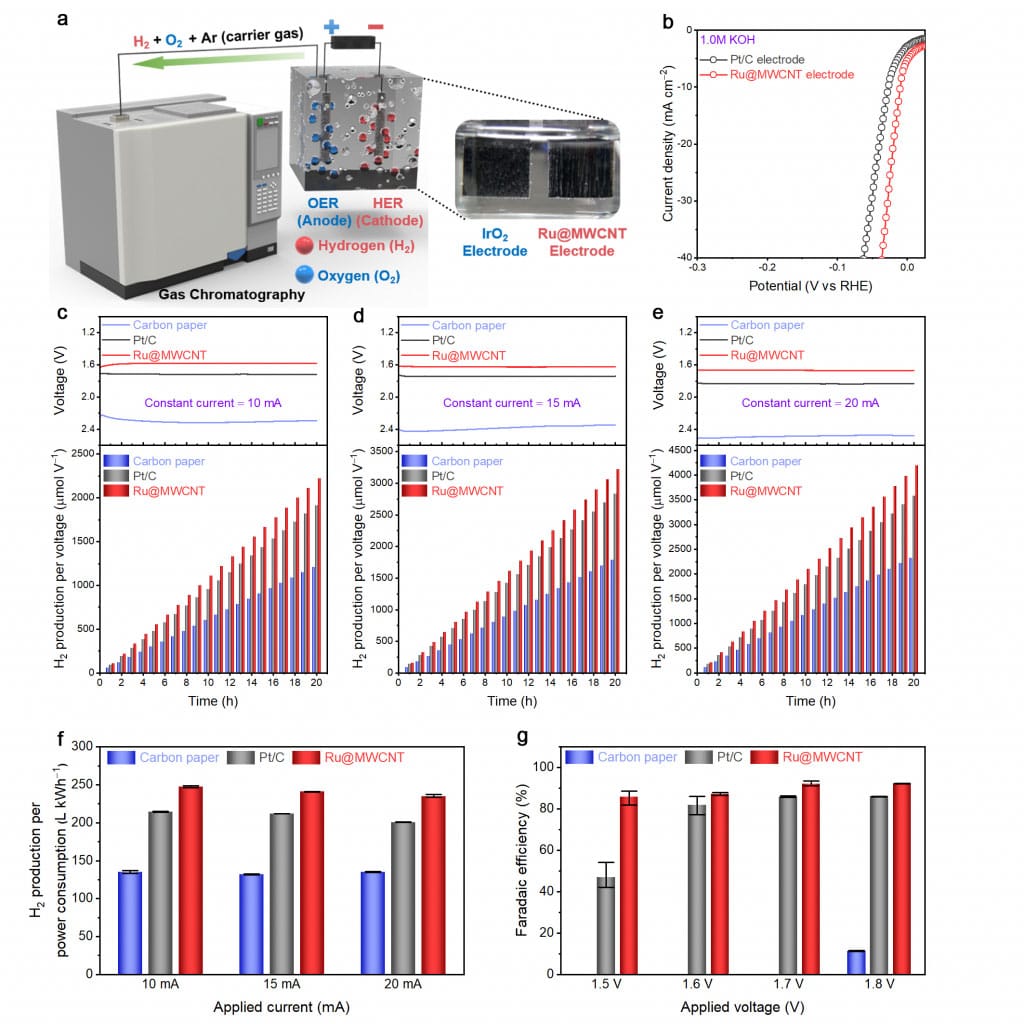
Scientists also determined the performance of the new catalyst by conducting the HER performance evaluation in the actual water-splitting system construction and analysis, in addition to the existing overpotential measurement. Their results show that Ru@MWCNT produces 15.4% more hydrogen per power consumption than commercial Pt/C, and Faradaic efficiency (92.28%) is higher than Pt/C (85.97%).
Professor Baek said, “Previous studies on hydrogen catalysts focus on the evaluation of the catalytic performance itself, and they were inadequate to deal with the actual water-splitting system construction and analysis. This study is significant as it can predict the actual HER applicability.”
Journal Reference:
- Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. DOI: 10.1038/s41467-020-15069-3