Alzheimer’s disease (AD) is the most common form of dementia worldwide. AD treatment has mainly focused on symptomatic treatment until recently, with the advent and approval of monoclonal antibody (MAB) immunotherapy.
A new study examines monoclonal antibody therapies for treating Alzheimer’s (AD). It cautions physicians to look for a potential side effect—amyloid-related imaging abnormalities (ARIA).
An accumulation of toxic amyloid-B is the major pathogenic aspect of AD. Monoclonal antibodies and other disease-modifying medications remove harmful amyloid-B protein from the brain.
Acuranumab (Aduhelm), as a treatment for AD, received fast approval from the U.S. Food and Drug Administration (FDA) in June 2021. According to the FDA, there is strong evidence that aducanumab lessens amyloid-B plaques in the brain, and patients are likely to benefit from this decrease.
Amit K. Agarwal, MBBS, MD, lead author of the article and neuroradiologist at Mayo Clinic in Jacksonville, FL, said, “FDA-approved drugs such as aducanumab, as well as upcoming newer-generation drugs, have provided an exciting new therapy focused on reducing the amyloid plaque burden in Alzheimer disease.”
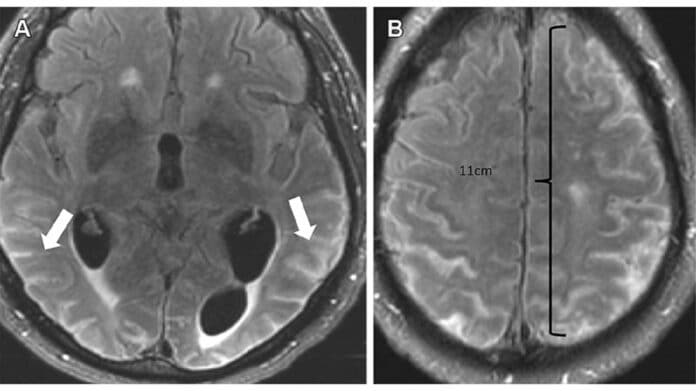
Although people with AD have benefited from this innovative new medication, some drawbacks exist. The identification of amyloid-related imaging abnormalities (ARIA) was made possible by the increased usage of monoclonal antibodies. The abnormalities have also been divided into two groups: ARIA-E, which stands for edema and/or effusion, and ARIA-H, which stands for hemorrhage. Increased vascular permeability following an inflammatory reaction, which results in the leakage of blood components and fluid into neighboring tissues, is thought to be the primary cause of ARIA.
Dr. Agarwal said, “Patients with ARIA sometimes have headaches, but they are usually asymptomatic and only diagnosable with MRI. It is essential for the radiologist to recognize and monitor ARIA.”
“As the use of monoclonal antibodies becomes more widespread, close collaboration between neurologists and radiologists is needed before and during therapy to plan for image monitoring per established guidelines.”
The most frequent adverse reaction to monoclonal antibody therapy is ARIA-E. 35% of participants receiving the recommended dose in two phase III trials got ARIA-E. These studies also revealed that 98% of ARIA-E patients resolved at follow-up imaging and that most ARIA-E cases were clinically asymptomatic.
The incidence of ARIA-E substantially decreased during the first nine months of treatment, peaking between three and six months of therapy. About 15–20% of patients receiving monoclonal antibodies get ARIA-H. ARIA-H is not transient and does not disappear with time, unlike ARIA-E.
Most patients with asymptomatic ARIA meeting specific radiographic and clinical criteria may continue to receive treatment. Most patients with ARIA-E can continue therapy either with or without temporary suspension. However, in ARIA-H patients, therapy decisions depend on the severity of ARIA-H and whether it is stabilized. The detection of 10 or more new microhemorrhages requires permanent discontinuation of therapy.
Dr. Agarwal said, “Immunotherapy is becoming more prevalent in managing dementia, and the recently approved monoclonal antibody therapy offers an exciting new frontier.”
“Identifying and monitoring ARIA plays a vital role in safety monitoring and management decisions in anti-amyloid monoclonal antibody trials and clinical practice.”
Dr. Agarwal states, “when ARIA is present, a conservative monitoring plan should be established with a multidisciplinary approach that includes neurologists and radiologists familiar with the clinical and imaging aspects of the condition.”
Journal Reference:
- Amit Agarwal, Vivek Gupta, Pavan Brahmbhatt, Amit Desai, Prasanna Vibhute, Nelly Joseph-Mathurin, Girish Bathla. Amyloid-related Imaging Abnormalities in Alzheimer Disease Treated with Amyloid-β Therapy. RadioGraphics, 2023; 43 (9) DOI: 10.1148/rg.230009