No matter which estimate we use, there are not enough ventilators for patients with Covid-19.
The coronavirus is straining the global health care system, and one piece of lifesaving medical equipment is in particularly scarce supply: mechanical ventilators.
Ventilators are machines that help patients breathe when they are unable on their own.
To deal with the shortage, a rapidly assembled volunteer team of engineers, physicians, computer scientists, and others, centered at MIT– called MIT E-Vent- is working to implement a safe, inexpensive alternative for emergency use, which could be built quickly around the world.
Students working in consultation with local physicians designed a simple ventilator device that could be built with about $100 worth of parts.
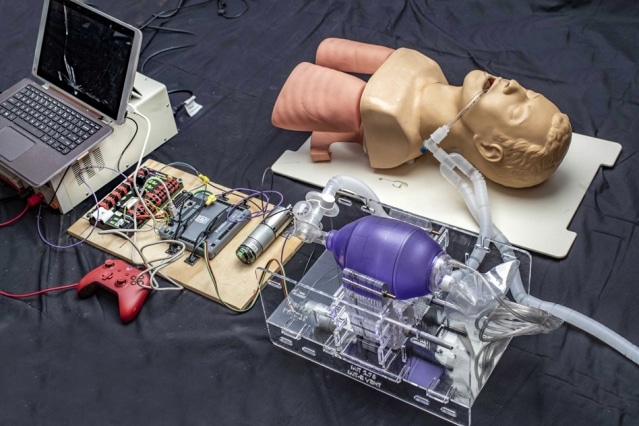
The ventilator delivers breaths by compressing a conventional bag-valve-mask (BVM) with a pivoting cam arm, eliminating the need for a human operator for the BVM.
An initial prototype was built out of acrylic, measuring 11.25 x 6.7 x 8 inches (285 x 170 x 200 mm) and weighing 9 lbs (4.1 kg). It is driven by an electric motor powered by a 14.8 VDC battery and features an adjustable tidal volume up to a maximum of 750 ml.
Tidal volume and number of breaths per minute are set via user-friendly input knobs. The prototype also features an assist-control mode and an alarm to indicate the over-pressurization of the system.
The team is particularly concerned about the potential for well-meaning but inexperienced do-it-yourselfers to try to reproduce such a system without the necessary clinical knowledge or expertise with hardware that can operate for days; around 1 million cycles would be required to support a ventilated patient over two weeks.
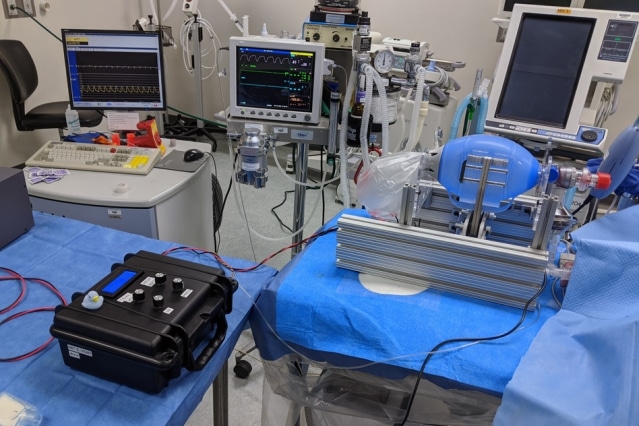
Furthermore, it requires a fault-tolerant code, since ventilators are precision devices that perform a life-critical function. To help curtail the spread of misinformation or poorly-thought-out advice, the team has added to their website verified information resources on the clinical use of ventilators and the requirements for training and monitoring in using such systems. All of this information is freely available at e-vent.mit.edu.
One of the team members said, “We are releasing design guidance (clinical, mechanical, electrical/controls, testing) on a rolling basis as it is developed and documented. We encourage capable clinical-engineering teams to work with their local resources, while following the main specs and safety information, and we welcome any input other teams may have.”
“This is not a project for typical do-it-yourselfers to undertake, since it requires specialized understanding of the clinical-technical interface, and the ability to work in consideration of strict U.S. Food and Drug Administration specifications and guidelines.”
“Such devices have to be manufactured according to FDA requirements, and should only be utilized under the supervision of a clinician. The Department of Health and Human Services released a notice stating that all medical interventions related to Covid-19 are no longer subject to liability, but that does not change our burden of care. At present, we are awaiting FDA feedback about the project. Ultimately, we intend to seek FDA approval. That process takes time, however.”
Journal Reference:
- Design and Prototyping of a Low-cost Portable Mechanical Ventilators. (PDF)
