For years, scientists have been fascinated by the mysterious phenomenon known as hydrogen spillover, which holds great potential for transforming the field of clean energy.
Essentially, hydrogen spillover occurs when small metal nanoparticles are anchored on a thermally stable oxide, like silica, comprising a major class of catalysts. While the catalytic reaction usually occurs on the reactive and expensive metal, on some catalysts, hydrogen atom-like equivalents literally spill from the metal to the oxide, forming what scientists term “hydrogen spillover.”
First described in 1964, however, it hasn’t gained much headway. While researchers have been able to identify hydrogen spillover for nearly 60 years, no one has been able to quantify it and describe the mechanism underpinning the phenomenon – until now.
In a recent breakthrough, a Penn State-led research team has discovered how and why hydrogen spillover occurs and provided the first quantitative measurement of the process. The work opens the door to better understanding and developing hydrogen activation and storage.
Conventional hydrogen storage requires significant amounts of energy to keep hydrogen in a liquid state. Now, researchers have developed a unique gold-on-titania system that can effectively, efficiently, and reversibly break apart hydrogen molecules into hydrogen atoms – a process needed to induce hydrogen spillover – at higher temperatures. This process requires significantly less energy when compared to conventional hydrogen storage.
According to researchers, hydrogen-spillover systems work by splitting hydrogen gas into hydrogen atom equivalents – a proton and an electron, but in a different arrangement than their usual layout. While the protons stick to the material’s surface, the electrons enter the semiconducting oxide’s near-surface conduction band. The researchers hope to utilize this system for testing advanced chemistry applications, such as converting atoms into clean fuels and hydrogen storage.
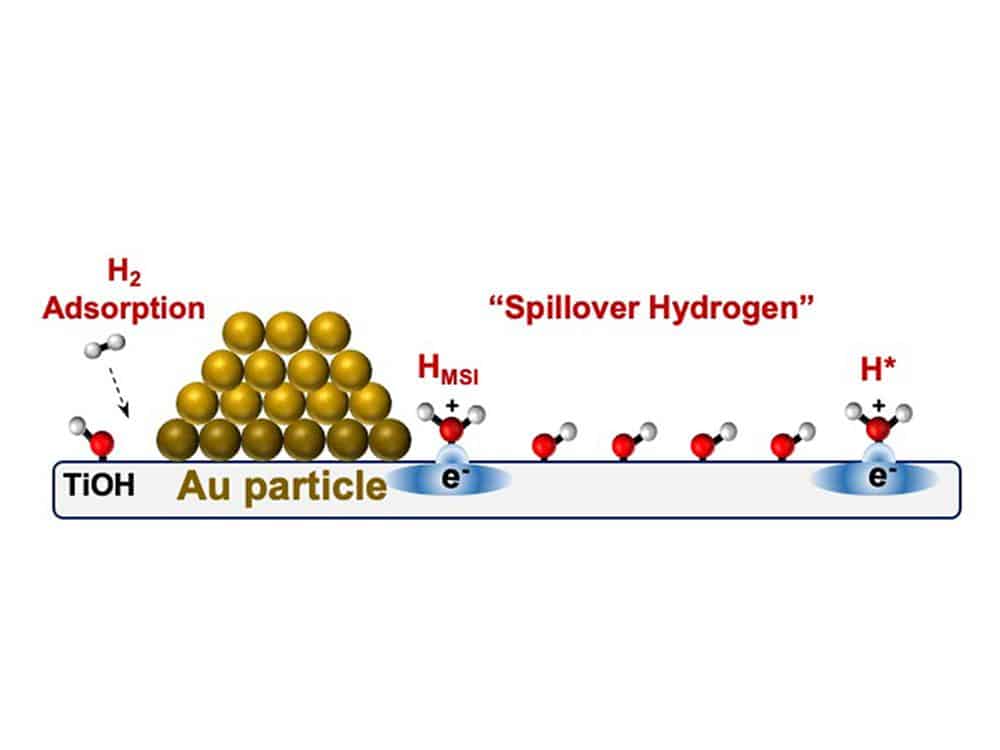
The research found that entropy plays a crucial part in driving the atoms from the metal to the substrate, providing a more nuanced understanding of the phenomenon.
Researchers had previously thought that hydrogen atom equivalents were strongly bonded to the nanoparticle layer and required significantly higher thermal energy to break those bonds and produce more spillover. However, most hydrogen spillover-facilitating systems tend to be complex, as the spillovers can appear to vary their bonding strength to both the nanoparticle and the semiconductor oxide substrate. This sticky bonding conceals true adsorption and masks what’s driving the spillover: thermal energy or entropy.
“We got really lucky with our choice of system, which we selected because we were already interested in how gold works as a catalyst,” Penn State professor Bert Chandler said, explaining that previous researchers could measure the amount adsorbed accurately because weak adsorption on the oxide masked the amount of spillover from the metal. “We didn’t invent new chemistry; we just collected the data. It took us six years of measuring and re-measuring – when you make an exceptional claim, you better have exceptional evidence – but we filled this hole in our understanding: entropy drives hydrogen spillover.”
Researchers are now planning to investigate material types that could facilitate better hydrogen storage. According to Chandler, the work is a step toward clean energy development and a striking example of how the scientific process works.
Journal reference:
- Akbar Mahdavi-Shakib, Todd N. Whittaker, Tae Yong Yun, K. B. Sravan Kumar, Lauren C. Rich, Shengguang Wang, Robert M. Rioux, Lars C. Grabow, and Bert D. Chandler. The role of surface hydroxyls in the entropy-driven adsorption and spillover of H2 on Au/TiO2 catalysts. Nature Catalysis, 2023; DOI: 10.1038/s41929-023-00996-3
