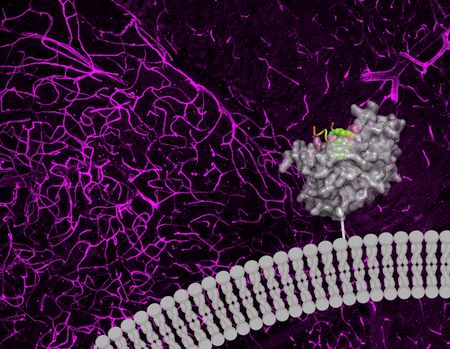
The blood-brain barrier (BBB) presents a significant challenge for delivering large molecules to study and treat the central nervous system. This is partly due to the scarcity of targets known to mediate BBB crossing.
A recently discovered mechanism by which some viral vectors—protein shells designed to deliver different desired cargo—can pass through the BBB has now been found by a new study from Caltech. This mechanistic understanding could offer a fresh perspective on creating viral vectors for research and medicine.
Even while the BBB provides a strong defense for the brain, some viruses have spontaneously acquired the ability to get beyond it. Researchers have been studying how to exploit these viruses as a type of BBB-crossing Trojan Horse for decades.
To achieve this, researchers scrape out the original viral payload the viruses carried and then use their hollow shells to ferry helpful treatments or instruments for research. Viral vectors crossing the BBB can transport targeted genes to the brain with just a little bloodstream injection, negating the need for invasive brain injection.
Unfortunately, the majority of naturally occurring virus-derived vectors are very ineffective at crossing the BBB, necessitating the administration of high doses and raising the possibility of side effects.
The directed evolution procedure, developed at Caltech by Nobel Laureate Frances Arnold, has been employed by the Gradinaru group during the past ten years to influence the evolution of vectors and improve their ability to cross the BBB.
The team has developed many vectors throughout the years, each with a unique ability to penetrate the BBB and target distinct tissues and cell types in a range of animals. They discovered along the way that many vectors can function differently in various model organisms, indicating that each of these vectors may have discovered unique and effective routes from the bloodstream to the brain.
However, although researchers knew these vectors could cross, it was still unclear how they crossed. Where are the entry points in the fortified wall of the BBB?
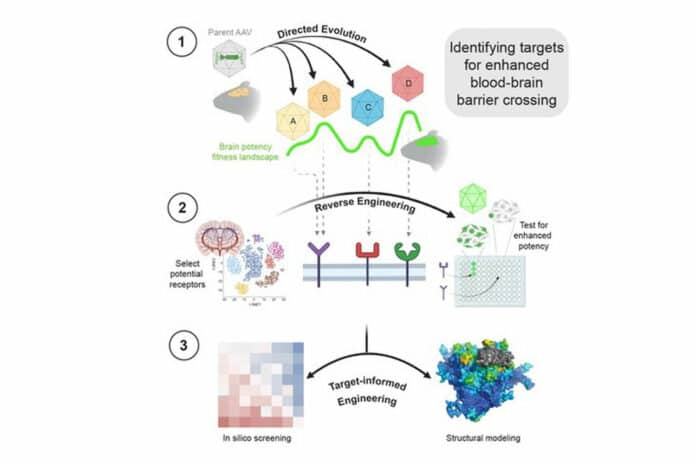
In this new study, scientists identified these mechanisms using a multidisciplinary approach that combines the researchers’ expertise in techniques of protein chemistry, molecular biology, and data science, respectively.
First, they developed a cell-culture screen to quickly test the ability of scores of diverse proteins found on the surface of the BBB to enhance the infectivity of vectors in a dish. They then used an advanced computational model (based on a complex artificial intelligence program called AlphaFold) to simulate how vectors interact with the different proteins, revealing the geometries of the interactions uncovered on the screen.
Next, a kind of “March Madness” competition process—which is the subject of a forthcoming paper—determined which vectors interacted best with which proteins and recapitulated the experimental results of the screen.
The team found that a specific enzyme known as carbonic anhydrase IV (CA-IV) allows a few different viral vectors to get through the BBB. It’s interesting to note that CA-IV, an ancient enzyme, is present on the BBBs of many different species, including humans.
Until recently, it was unknown whether CA-IV promoted BBB-crossing. The team is enthusiastic about the potential to use these molecular gateways to deliver brain medicines and believes that this combination of experimental and computational strategy may speed up the discovery of further BBB crossing solutions.
Blood-brain-barrier crossing is a key biological puzzle,” says Gradinaru. “To say that an enzyme that regulates blood pH and lets us taste the fizz in soda is an unintuitive target for helping viruses through the BBB would be an understatement. Now we can leverage CA-IV, and other exciting targets that continue to emerge from our approach rooted in identifying the mechanisms of BBB-crossing viral vectors, to help us design next-generation viral and non-viral delivery vectors for the brain. And maybe, it will also help us build resilience against emergent pathogens that could hijack the same routes for brain entry.”
Journal Reference:
- Timothy Shay, Erin Sullivan, et al. Primate-conserved carbonic anhydrase IV and murine-restricted LY6C1 enable blood-brain barrier crossing by engineered viral vectors. Science Advances. DOI: 10.1126/sciadv.adg6618