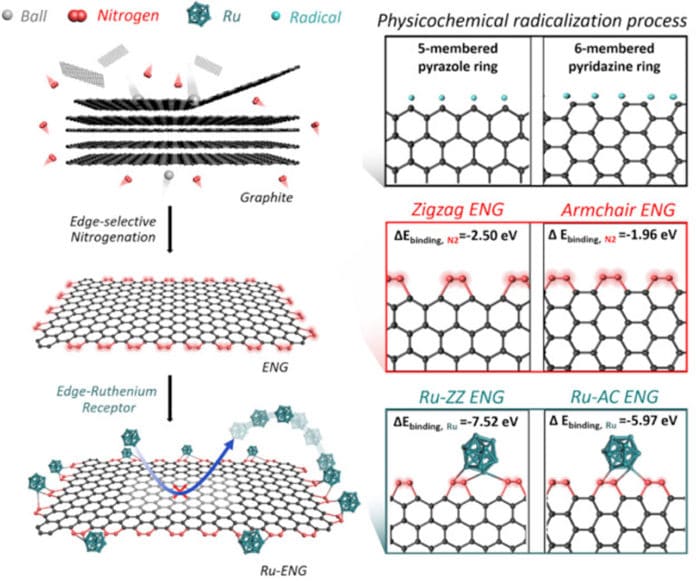
A research team at UNIST has developed a new class of efficient catalysts that show outstanding stability regardless of electrolyte pH levels during electrochemical responses, such as water splitting.
Made from inexpensive carbon compounds and ruthenium (Ru), the catalysts are stable and display efficient catalytic activity and improved electrochemical performances.
Pure water is a bad conductor of electricity as it contains very few ions. Therefore, adding acids or bases to water increases the concentration of ions in the solution, thereby causing an electrochemical reaction. Catalysts reduce the energy needed to make a chemical reaction take place. The decreased energy consumption that this causes increases the catalyst efficiency.
Hence, to accelerate the commercial use of different electrochemical reactions, there is a requirement for catalysts that show high catalytic efficiency with high durability. Platinum (Pt) has been generally utilized in various industries because of its high electrocatalytic movement. However, simultaneously, it is additionally exceptionally corrosive in alkaline media, subsequently bringing about poor durability.
In this new study, scientists reported Ru nanoparticles anchored at edge-selectively nitrogenated graphitic nanoplatelets (Ru-ENG) instead of on the basal plane in two-dimensional (2D) graphitic substrate.
Yejin Yang (Combined MS/Ph.D. program of Energy and Chemical Engineering, UNIST), the first author of the study, said, “Our work represents the edge-sites design of graphitic nanoplatelets with Ru nanoparticles for an efficient and stable HER electrocatalyst at universal pH.”
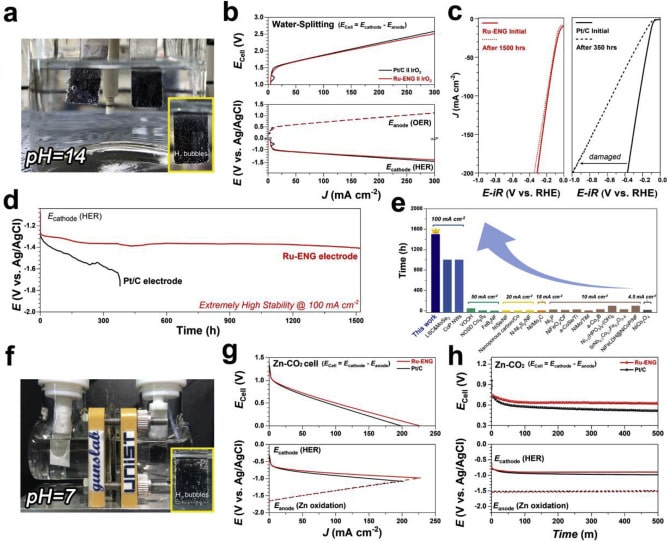
Scientists evaluated the catalyst’s stability by performing accelerated degradation studies in all pH conditions. Their outcomes suggest that the proposed Ru-ENG catalyst showed much better electrochemical performance and stability compared to commercial Pt/C in universal pH conditions. It also indicates superior long-term consecutive stability (over 1,500 h) and unusual hydrogen evolution activities in practical applications.
Jeongwon Kim (Combined MS/Ph.D. program of Energy and Chemical Engineering, UNIST), the first co-author of the study, said, “Notably, the current density value of Ru-ENG catalyst for the hydrogen generation efficiency in Aqueous Zn-CO2 system was also higher than that of Pt-based catalyst.”
“This work provides a potentially powerful approach for the design of rationally selective heteroatom substitution with other active metals as a promising HER candidate regardless of pH condition.”
Journal Reference:
- Yejin Yang, Jeongwon Kim, Changmin Kim, et al., “Edge-selective decoration with ruthenium at graphitic nanoplatelets for efficient hydrogen production at universal pH,” Nano Energy, (2020). DOI: 10.1016/j.nanoen.2020.105114