Sepsis is a conceivably life-threatening condition caused by the body’s reaction to an infection. The body normally discharges chemicals into the bloodstream to battle an infection. Sepsis happens when the body’s reaction to these infections is out of balance, activating changes that can harm various organ systems.
To diagnose sepsis, doctors traditionally rely on various diagnostic tools, including vital signs, blood tests, and other imaging and lab tests.
MIT scientists have devised a sensor that can drastically speed up the sepsis’s diagnosing process. The sensor is originally a microfluidics-based system that automatically detects clinically significant levels of IL-6, a promising candidate in Sepsis, within just 25 minutes.
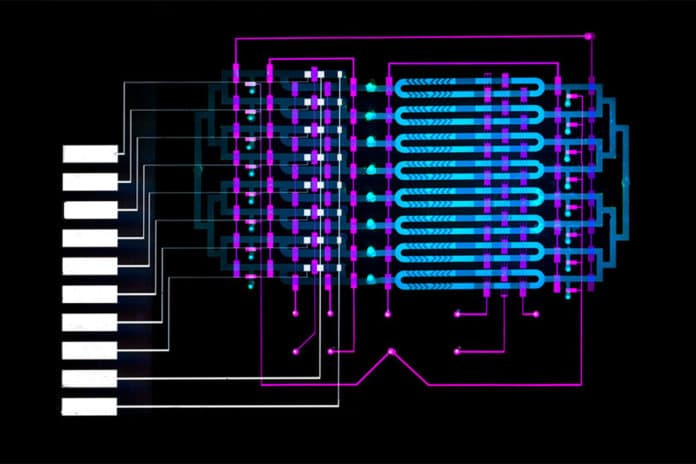
In their work, the researchers wanted to shrink components of the magnetic-bead-based assay, which is often used in labs, onto an automated microfluidic device that’s roughly several square centimeters. That required manipulating beads in micron-sized channels and fabricating a device in the Microsystems Technology Laboratory that automated the movement of fluids.
The beads are coated with an antibody that attracts IL-6, as well as a catalyzing enzyme called horseradish peroxidase. The beads and blood sample are injected into the device, entering into an “analyte-capture zone,” which is basically a loop. Along the loop is a peristaltic pump — commonly used for controlling liquids — with valves automatically controlled by an external circuit. Opening and closing the valves in specific sequences circulates the blood and beads to mix together. After about 10 minutes, the IL-6 proteins have bound to the antibodies on the beads.
Automatically reconfiguring the valves around then forces the mixtures into a smaller loop, called the “detection zone,” where they remain caught. A tiny magnet gathers the beats for a brief wash before releasing them around the loop. After around 10 minutes, numerous dots have stuck on a cathode covered with a different immune response that pulls in IL-6. Around then, an answer streams into the circle and washes the untethered globules, while the ones with IL-6 protein stay on the terminal.
The arrangement conveys a particular atom that responds to the horseradish protein to make an aggravate that reacts to power. At the point when a voltage is connected to the arrangement, each outstanding dot makes a small current. A common chemistry strategy called “amperometry” changes over that current into a clear sign. The gadget checks the sign and figures the grouping of IL-6.
First author Dan Wu, a Ph.D. student in the Department of Mechanical Engineering said, “On their end, doctors just load in a blood sample using a pipette. Then, they press a button and 25 minutes later they know the IL-6 concentration.”
The device uses about 5 microliters of blood, which is about a quarter the volume of blood drawn from a finger prick and a fraction of the 100 microliters required to detect protein biomarkers in lab-based assays. The device captures IL-6 concentrations as low as 16 picograms per milliliter, which is below the concentrations that signal sepsis, meaning the device is sensitive enough to provide clinically relevant detection.
Scientists are further planning to create a panel of important sepsis biomarkers for the device to capture, including interleukin-6, interleukin-8, C-reactive protein, and procalcitonin.
Wu said, “This is a very general platform. If you want to increase the device’s physical footprint, you can scale up and design more channels to detect as many biomarkers as you want.”