Even though battery technology has certainly improved over the last century. Much research and development are being done on battery technology to improve performance while ensuring that batteries are lightweight, compact, and affordable.
Lithium-sulfur batteries (LI-S) hold the potential to revolutionize the rechargeable battery market. Li-S offers a theoretical energy density more than five times that of lithium-ion batteries.
Now, Scientists at the Chalmers University of Technology, Sweden have reported a novel, simple, and environmentally benign synthesis route for this type of battery, using a catholyte with the help of a graphene sponge.

“Lithium sulfur batteries have the advantage of not needing to contain any environmentally harmful fluorine, as is commonly found in lithium ion batteries,” says Aleksandar Matic, Professor of Condensed Matter Physics.
CREDIT
Johan Bodell/Chalmers University of Technology
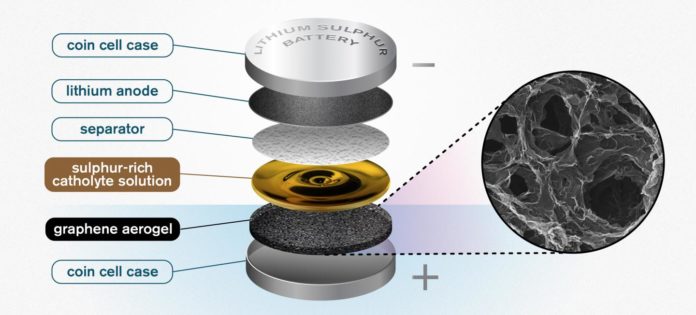
Their new idea is a porous, sponge-like aerogel, made of reduced graphene oxide, that acts as a free-standing electrode in the battery cell and allows for better and higher utilization of sulfur. Scientists began with experimenting and combining the cathode and electrolyte into one liquid, so-called catholyte. Doing this can make battery lightweight and offers fast charging functionality with better power capability. ANd adding aerogel to this could make this concept viable with additional promising results.
Carmen Cavallo of the Department of Physics at Chalmers said, “You take the aerogel, which is a long thin cylinder, and then you slice it – almost like a salami. You take that slice, and compress it, to fit into the battery. hen, a sulfur-rich solution – the catholyte – is added to the battery. The highly porous aerogel acts as the support, soaking up the solution like a sponge.”
“The porous structure of the graphene aerogel is key. It soaks up a high amount of the catholyte, giving you high enough sulfur loading to make the catholyte concept worthwhile. This kind of semi-liquid catholyte is really essential here. It allows the sulfur to cycle back and forth without any losses. It is not lost through dissolution – because it is already dissolved into the catholyte solution.”

CREDIT
Johan Bodell/Chalmers University of Technology
“Some of the catholyte solutions is applied to the separator as well, in order for it to fulfill its electrolyte role. This also maximizes the sulfur content of the battery.”
Sulfur represents a natural cathode partner for metallic Li and, in contrast with conventional lithium-ion cells, the chemicals processes include dissolution from the anode surface during discharge and reverse lithium plating to the anode while charging. As a consequence, Li-S allows for theoretical specific energy in excess of 2700Wh/kg, which is nearly 5 times higher than that of Li-ion.
The Aleksandar Matic, Professor at Chalmers Department of Physics, who leads the research group behind the paper said, “The problem with lithium-sulfur batteries so far has been their instability and consequent low cycle life. Current versions degenerate fast and have a limited life span with an impractically low number of cycles. But in the testing of their new prototype, the Chalmers researchers demonstrated an 85% capacity retention after 350 cycles.”
The new design avoids the two main problems with the degradation of lithium-sulfur batteries – one, that the sulfur dissolves into the electrolyte and is lost, and two, a ‘shuttling effect’, whereby sulfur molecules migrate from the cathode to the anode. In this design, these undesirable issues can be drastically reduced.
Matic said, “However, that there is still a long journey to go before the technology can achieve full market potential. Since these batteries are produced in an alternative way from most normal batteries, new manufacturing processes will need to be developed to make them commercially viable.”
The study is published in the Journal of Power Sources.