Japanese researchers have successfully generated electricity directly from methylcyclohexane, an organic hydride, using solid oxide fuel cells (SOFC) with lower energy than conventional catalytic dehydrogenation reactions.
Power generation by fuel cells that use hydrogen is one of the key technologies for realizing a low-carbon society. It is abundant and versatile and emits only water when used as a fuel.
However, in order to spread a hydrogen society, it is just as important to develop hydrogen infrastructure as it is to develop fuel cells. There are various hydrogen carriers that are currently being investigated for this purpose, including high-pressure hydrogen gas, liquid hydrogen, ammonia, formic acid, and organic hydrides.
In particular, methylcyclohexane (MCH), one of the organic hydrides, is expected to be a great hydrogen carrier that can safely and efficiently transport and store hydrogen. Dehydrogenation of MCH under the presence of a catalyst produces hydrogen and toluene as a byproduct, and then electricity is generated from that hydrogen.
However, the dehydrogenation process is an endothermic reaction, and irrecoverable energy loss, as well as the facilities required for the reaction, are issues.
Recently, researchers at the Waseda University in Japan have succeeded in using SOFC to generate electricity directly from methylcyclohexane while also recovering toluene so it can be reused. This research is expected to not only reduce energy requirements but also open up new possibilities for chemical synthesis using fuel cells.
The research team attempted to perform two processes simultaneously in a fuel cell: dehydrogenation from organic hydrides, an endothermic reaction, and electricity generation, an exothermic reaction. To achieve this, they used an anode-supported solid oxide fuel cell that operated at a higher temperature than a polymer electrolyte fuel cell.
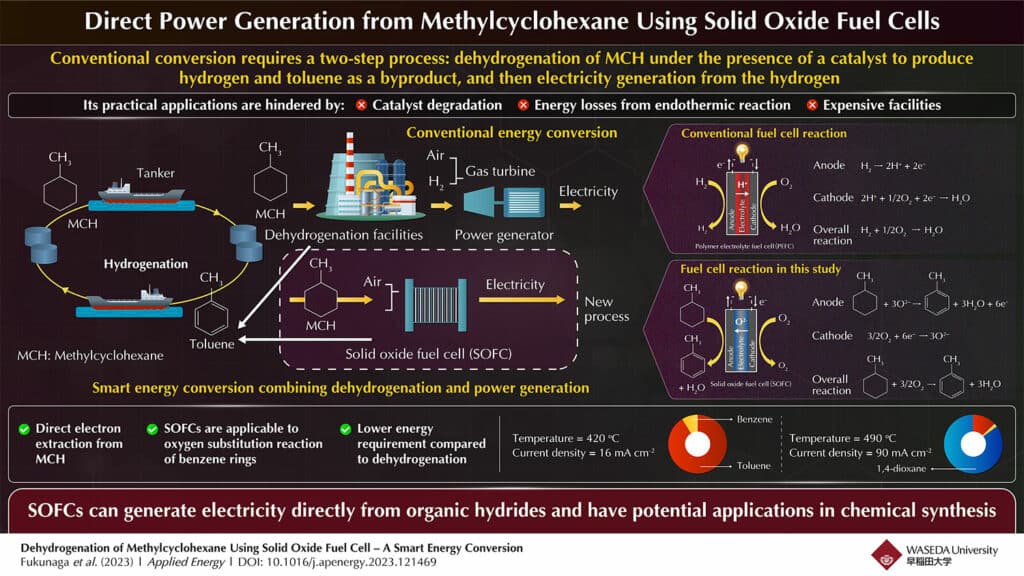
The team was able to operate it at a temperature that did not allow pyrolysis of organic hydrides and under conditions that prevented carbon deposition at the electrodes. They reported that the production ratio of toluene to benzene was 94:6, which is a significant accomplishment.
These findings indicate that an SOFC successfully extracts electrons directly from MCH and generates electricity by utilizing the SOFC’s oxygen ion conduction function. This method shows the potential for electricity generation without the need for dehydrogenation facilities and with less energy required than conventional catalyst-assisted MCH dehydrogenation.
“Fuel cells have been studied and developed as devices that produce highly efficient, carbon-free electricity through the electrochemical reaction of hydrogen and oxygen. In this study, we have demonstrated that this device can be applied to control dehydrogenation reactions from organic hydrides and oxygen substitution reactions of aromatic rings. In the future, new synthetic chemistry may be created by applying fuel cells,” concludes Professor Akihiko Fukunaga, who led the research.
Journal reference:
- Akihiko Fukunaga, Asami Kato, Yuki Hara, Takaya Matsumoto. Dehydrogenation of methylcyclohexane using solid oxide fuel cell – A smart energy conversion. Applied Energy, 2023; DOI: 10.1016/j.apenergy.2023.121469
