In 1869, Mendeleev proposed the periodic table of the elements, which has long been the foundation of the natural sciences. The periodic table has contributed to the discovery of a number of the elements that obey a certain periodic rule.
Could there be such a periodic table for molecules?
Some scientists think- it is possible and proposed periodic rules for predicting the existence of certain molecules, these predictions were valid only for clusters of atoms with a quasi-spherical symmetry, because of the limitations of their own theory. Thus, scientists proposed a new approach to build a periodic table for molecules with multiple types of symmetries.
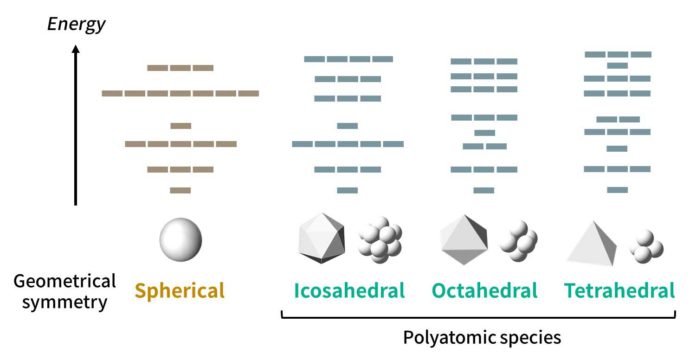
Scientists observed the behaviour of the valence electrons of atoms that form molecular clusters. When numerous atoms form a cluster with a symmetrical shape, their valence electrons tend to occupy specific molecular orbitals called as “super-atomic orbitals,” in which they behave on precisely as though they were the electrons of a huge atom.
By thinking about this fact and analyzing the impacts of the structural symmetries for clusters, scientists proposed “symmetry-adapted orbital (SAO) models,” which are in agreement with various known molecules just as best in class quantum-mechanical calculations.
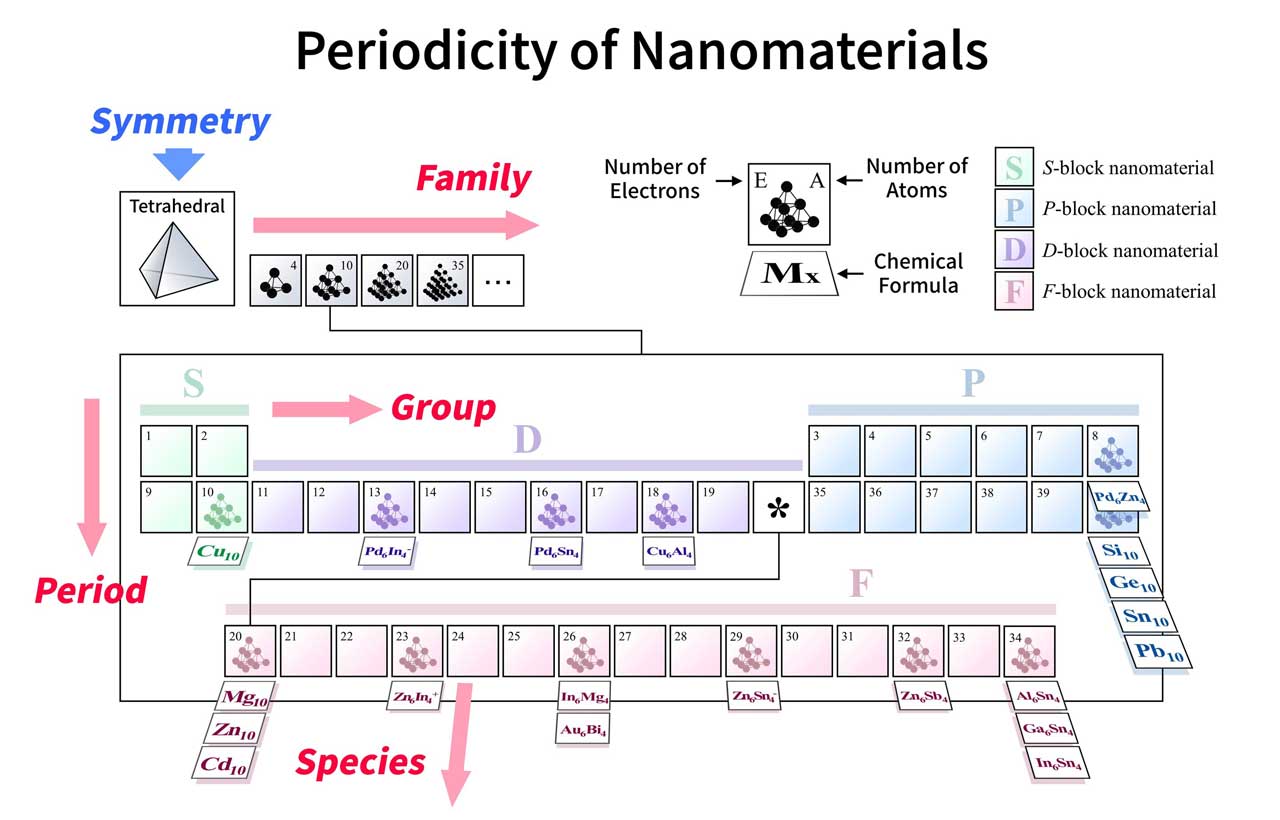
The new periodic tables, which would be created for each symmetry type, would actually be four-dimensional, because the molecules would be arranged according to four parameters: groups and periods (based on their “valence” electrons, similar to the normal periodic table), species (based on the constituting elements), and families (based on the number of atoms).
Prof. Kimihisa Yamamoto said, “The SAO approach is very promising in the field of materials design. Modern synthesis techniques enable us to produce many innovative materials based on the SAO model, such as lightweight magnetic materials.”
“The road ahead for scientists lies in further expanding these tables to molecular clusters with other shapes and symmetries and predicting stable molecules that have yet to be developed.”
“Among the infinite combinations of constitutive elements, the proposed periodic table will be a significant contribution to the discovery of novel functional materials.”
The study is published in the journal Nature Communications.