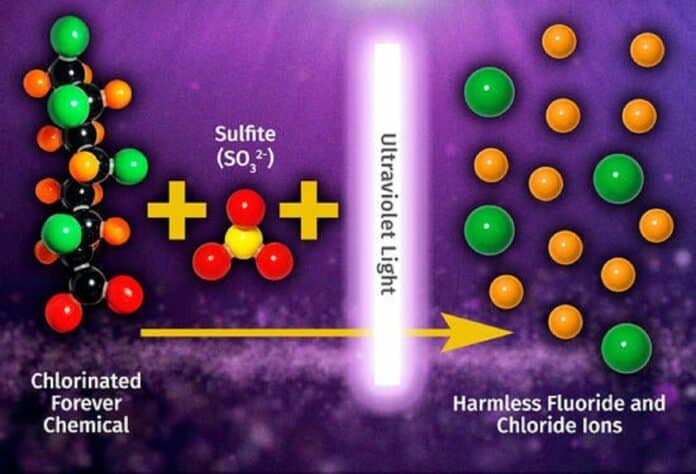
The University of California, Riverside (UCR) researchers have discovered a chemical reaction mechanism that can degrade chlorinated ” forever chemicals” into harmless substances.
The researchers employed UV light and sulfite to break chlorine-to-carbon bonds and carbon-to-carbon and carbon-to-fluorine bonds, crucial for the quick and near-complete defluorination of PFAS compounds required for contamination cleanup efforts.
PFAS can be found in many products, including potato chip bags, stain and water repellents, cleaning products, nonstick cookware, and fire-fighting foams. Because of their fluorine-to-carbon solid chemical connections, they can survive in the environment for decades or longer.
Forever chemicals, also known as poly- and per-fluoroalkyl compounds, have been utilized in thousands of items ranging from potato chip bags to stain and water repellents used on fabrics, cleaning products, nonstick cookware, and fire-suppressing foams. Because of their fluorine-to-carbon solid chemical connections, they can survive in the environment for decades or longer.
Chlorinated PFAS is a vast set of thousands of chemicals in the Forever chemical family. They include non-flammable hydraulic fluids used in industry and compounds used to create chemically stable films that serve as moisture barriers in various industrial, packaging, and electronic applications.
Recent findings of previously identified chlorinated PFAS contaminants in the environment have raised additional worries, and the EPA is now enacting new laws to accelerate cleanup efforts across the country.
All around the country, chemicals are getting into groundwater supplies, and they have been connected to various negative health outcomes, including cancer, kidney illness, and hormonal changes. The EPA is announcing new regulations to encourage cleanups across the country.
The team is investigating additional methods and mechanisms for PFAS degradation, and its ultimate objective is to break all carbon-fluorine bonds to detoxify PFAS contaminants.
In Liu’s investigation, the chlorine-to-carbon bonds were broken using sulfite and UV radiation. In addition to cleaving carbon-to-carbon and carbon-to-fluorine bonds, this triggered a series of chemical processes essential for the quick and nearly complete defluorination of PFAS compounds required for contamination cleanup efforts.
Liu said, “Our team is examining more pathways and strategies for PFAS degradation, and our ultimate goal is to cut all of the carbon-fluorine bonds to detoxify the PFAS pollutants completely.”
Liu’s recent findings on chlorinated PFAS compounds built on his team’steam’s discoveries in 2016 that used a combination of UV light and sulfite to eliminate different PFAS contaminants in badly contaminated water. Seven scientific journal publications have now been published detailing these procedures. They offer promise because sulfite is a low-cost molecule used as a food preservative, and ultraviolet radiation is currently employed to destroy potentially hazardous microorganisms in tap water treatment facilities.
This process of PFAS degradation generates fluorine ions, which are widely added to municipal water sources to improve oral health.
The industry is noticing Jinyong Liu’s work on PFAS compound removal. His organization has collaborated with three companies interested in expanding his laboratory work to perform cleanups at US Air Bases and Airports under federal contracts.
Spills of fire-suppressing foams, used for decades to quell aviation fuel fires, are a significant source of PFAS pollution in groundwater and below military air bases and commercial airports. These foams, developed by the US Navy in the 1960s, build an aqueous coating around the burning liquid, swiftly depriving it of oxygen and extinguishing it.
Journal Reference:
- Gao, J., Liu, Z., Chen, Z. et al. Photochemical degradation pathways and near-complete defluorination of chlorinated poly-fluoroalkyl substances. Nature Water. DOI: 10.1038/s44221-023-00046-z