For quite a long time, researchers have hunt down compelling approaches to remove overabundance carbon dioxide emissions from the air and reuse them into items, for example, renewable fuels. Be that as it may, the way toward changing over carbon dioxide into useful chemical is monotonous, costly, and inefficient and in this manner not financially or naturally viable.
Now, a new study by the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) in collaboration with Joint Center for Artificial Photosynthesis (JCAP) suggests that recycling carbon dioxide into valuable chemicals and fuels can be economical and efficient. And it is possible by using a single copper catalyst.
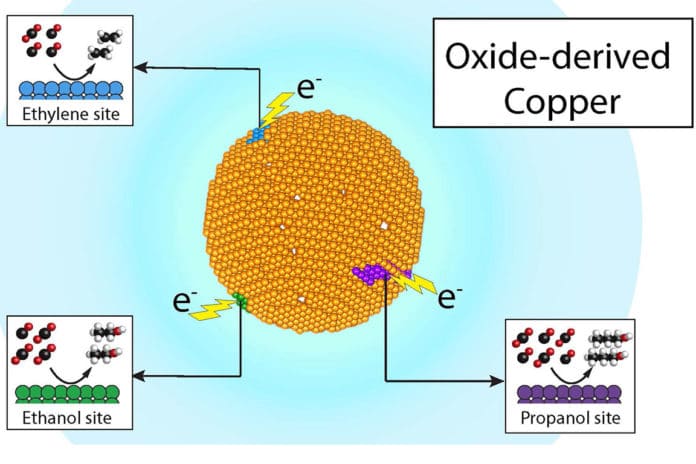
Joel Ager, a researcher at JCAP who led the study, said, “When you take a piece of copper metal, it may feel smooth to the touch, but at the microscopic level, the surface is actually bumpy – and these bumps are what scientists call active sites.”

Previous studies had shown that “oxidized” or rusted copper is an excellent catalyst for making ethanol, ethylene, and propanol. The researchers theorized that if active sites in copper were actually product-specific, they could trace the chemicals’ origins through carbon isotopes, “much like a passport with stamps telling us what countries they visited
Scientists ran a series of experiments by using two isotopes of carbon-carbon 12 and carbon-13. They labeled carbon-12 with carbon dioxide and carbon-13 with carbon monoxide. According to their methodology, the researchers reasoned that the ratio of carbon-13 versus carbon-12 – the “isotopic signature” – found in a product would determine from which active sites the chemical product originated.
After running dozens of experiments and used state-of-the-art mass spectrometry and NMR (nuclear magnetic resonance) spectroscopy at JCAP to analyze the results, they found that three of the products – ethylene, ethanol, and propanol – had different isotopic signatures showing that they came from different sites on the catalyst.
Co-author Yanwei Lum, who was a UC Berkeley Ph.D. student said, “This discovery motivates future work to isolate and identify these different sites. Putting these product-specific sites into a single catalyst could one day result in a very efficient and selective generation of chemical products.”
Using the method, scientists are hoping to create green chemical manufacturing where a solar cell could feed electrons to specific active sites within a copper catalyst to optimize the production of ethanol fuels.
The work appears in the Dec. 17 edition of the journal Nature Catalysis.