Urinary Tract infection (UTI), the most common bacterial infection in any part of the urinary system. Doctors typically treat urinary tract infections with antibiotics.
In most cases, UTIs are caused by viruses or fungi such as Escherichia coli or E. coli. The E. Coli bacterium infects cells that line the bladder wall and form what is known as “intracellular bacterial communities.”
The “communities” repeatedly rupture, and bacteria re-enter neighboring cells, ultimately killing off the alleged “umbrella cells” that line the furthest layer of the bladder’s epithelium. The deficiency of umbrella cells then, at that point, permits the microorganisms to attack the more profound layers of the bladder, where they can form “quiescent intracellular reservoirs” that are impervious to antibiotics and cause UTI recurrences. The elements of these occasions are difficult to catch in vivo in animal models.
Kunal Sharma, the lead author on the two studies, said, “Infection dynamics are difficult to capture from static imaging of tissue explants at serial time points. Thus far, in vitro models have not recapitulated bladder architecture with sufficient fidelity to study the time course of these events.”
To address this, EPFL scientists have developed two complementary bladder models to study urinary tract infections (UTIs) caused by E. coli: bladder organoids and a bladder-on-a-chip. The first model recreates the 3D stratified architecture of the bladder epithelium. On the other hand, the second model incorporates physiological stimuli, e.g., the mechanical effect of bladder filling and voiding, and an interface with the vasculature to study immune cell migration to sites of infection.
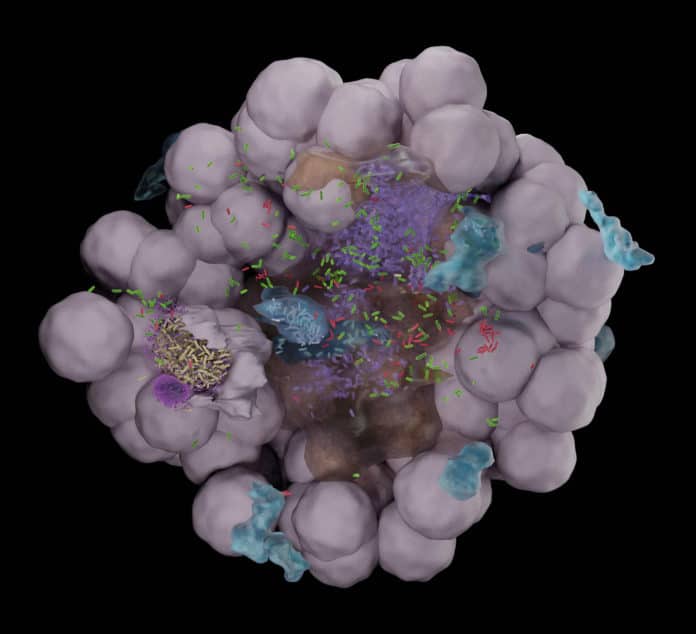
The organoids were generated from a mouse with a fluorescent label incorporated within cell membranes. Doing so enabled scientists to identify specific bacterial niches within the organoid with a high spatial resolution.
Sharma said, “By multiple imaging organoids, we managed to identify heterogeneity and diverse outcomes of host-pathogen interactions. This proof-of-concept system has shown promising potential for follow-up studies on bacterial persistence to antibiotics and the dynamics of immune cell responses to infection.”
Scientists later used volumetric electron microscopy and found that the bacteria invade deeper layers of the bladder, independently of the formation of intracellular bacterial communities, where they are protected from antibiotics and host immune cells.
Using a complementary bladder-on-chip model, scientists observed the growth dynamics of bacteria within intracellular bacterial communities over time. They developed human umbrella and endothelial cells together under a simulated urine-flow system and applied mechanical stresses to mimic the natural expansion and contraction of the bladder.
When scientists focused on the role of neutrophil recruitment in response to infection, they found that neutrophils cannot reduce intracellular bacterial communities. After tracking intracellular bacterial communities over successive cycles of antibiotic treatment, scientists found them to be highly dynamic and resistant to antibodies.
McKinney said, “These studies are part of the NCCR-funded “AntiResist” consortium aimed at developing more realistic in vitro models for infectious diseases, and using this knowledge to develop optimal treatment strategies, which could potentially have an enormous impact on human health.”
Vivek V. Thacker, a senior author on both studies, said, “Microphysiological models bridge the gap between simple cell culture systems and animal models. The two models complement each other well and are tailored to study specific aspects of the disease. We hope they will serve as a resource for the wider microbiology community and advance the synergies between the tissue engineering and infectious diseases communities.”
Journal Reference:
- Kunal Sharma, Neeraj Dhar, Vivek V Thacker, Thomas M. Simonet, Francois Signorino-Gelo, Graham William Knott, John D McKinney. Dynamic persistence of UPEC intracellular bacterial communities in a human bladder-chip model of urinary tract infection. eLife 05 July 2021;10:e66481 DOI: 10.7554/eLife.66481
- Kunal Sharma, Vivek V. Thacker, Neeraj Dhar, Maria Clapés Cabrer, Anaëlle Dubois, François Signorino-Gelo, Jasper Mullenders, Graham Knott, Hans Clevers, John D. McKinney. In an organoid model, early invasion of the bladder wall by solitary bacteria protects uropathogenic E. coli from antibiotics and neutrophil swarms. Cell Reports 20 July 2021. DOI: 10.1016/j.celrep.2021.109351