Nitrogen is a hot item in the surface sea. Essential makers including phytoplankton and different microorganisms devour and change it into natural particles to construct biomass, while others change inorganic structures to get to their compound store of vitality. These means are a piece of the perplexing nitrogen cycle of the upper water section.
Around 200 meters down, just beneath the sea’s sunlit zone, live a layer of nitrite, a middle of the road compound in the nitrogen cycle. Researchers have discovered this hearty element, called the essential nitrite most extreme, all through the world’s oxygenated seas. While a few individual theories have been advanced, none have convincingly clarified this marine mark as of not long ago.
A new study by the MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS) discovered the ecological mechanisms producing the observed nitrate accumulation and dictating its location in the water column.
Regardless of its low maritime focus, nitrite (NO2-) assumes a key part in worldwide carbon and nitrogen cycles. The majority of the nitrogen in the sea dwells in the inorganic type of nitrate (NO3-), which essential makers and microorganisms synthetically lessen it to construct natural particles.
Remineralization happens when the invert procedure happens: Phytoplankton and other heterotrophic microscopic organisms separate these natural mixes into ammonium (NH4+), a type of inorganic nitrogen. Ammonium at that point can be devoured again by essential makers, which get their vitality from light.
Different microorganisms called chemoautotrophs additionally utilize the ammonium both to make new biomass and as a wellspring of vitality. To do this, they remove oxygen from seawater and change it, a procedure called nitrification, which happens in two stages. To start with, the organisms change over ammonium into nitrite and after that to nitrate.
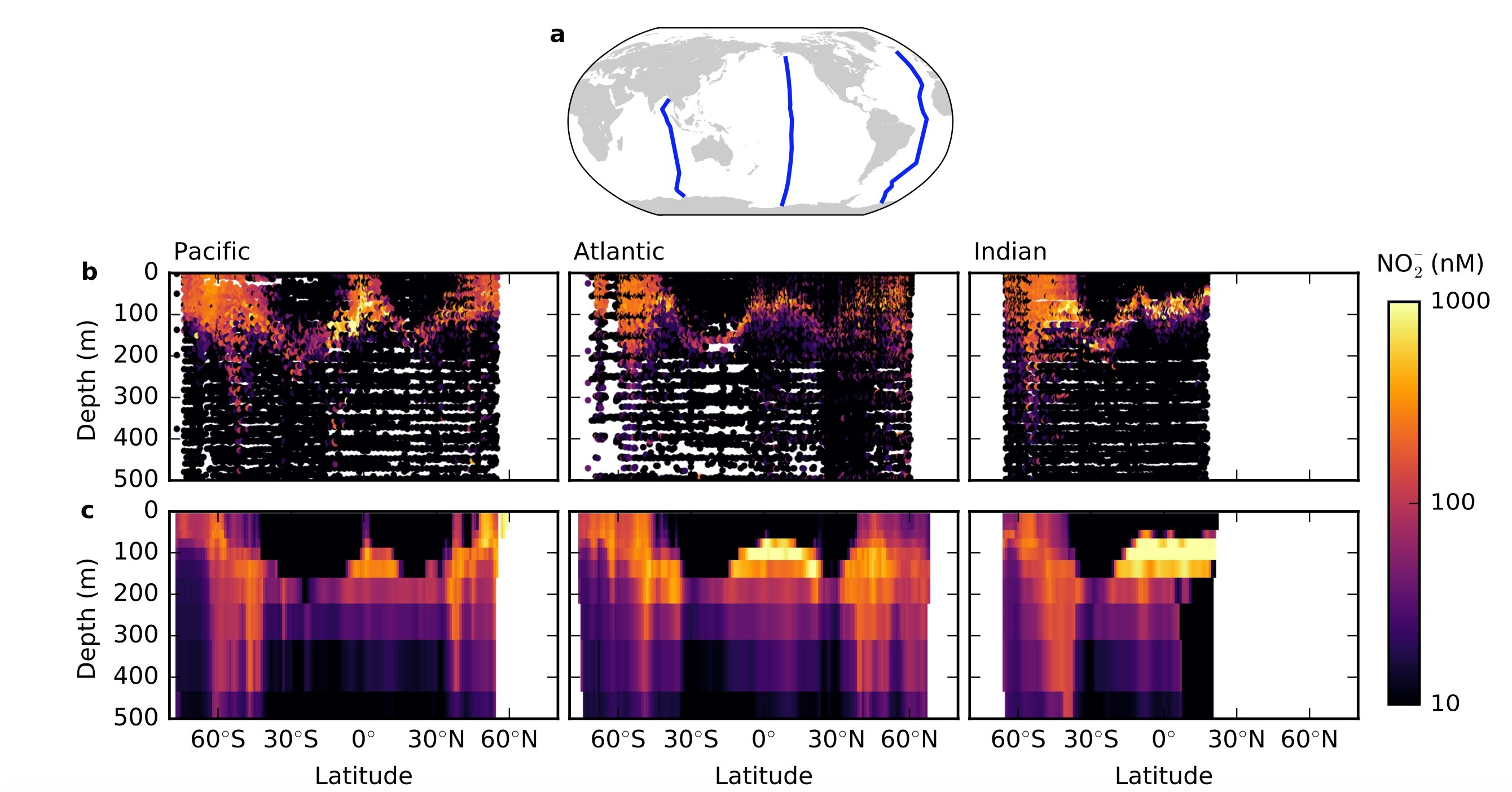
Image courtesy of the researchers.
Lead author Emily Zakem — a former EAPS graduate student who is now a postdoc at the University of Southern California — along with EAPS Principal Research Scientist Stephanie Dutkiewicz and Professor Mick Follows show that physiological constraints and resource competition between phytoplankton and nitrifying microorganisms in the sunlit layer can yield this ocean trait.
Zakem said, “Somewhere along the line, nitrite has been accumulating at the base of the sunlit zone, which has implications for ocean biogeochemistry. Broadly, we’re trying to understand what controls the remineralization of organic matter in the ocean. It’s that remineralization that is responsible for forming the biological pump, which is the extra storage of carbon in the ocean due to biological activity.”
“It’s this strong influence that nitrogen has on the global carbon cycle that captures Follows’ interest. The growth of phytoplankton on nitrate is called ‘new production’ and that balances the amount that’s sinking out of the surface and controls how much carbon is stored in the ocean. The growth of phytoplankton on ammonium is called recycled production, which does not increase ocean carbon storage.”
“So we wish to understand what controls the rates of supply and relative consumption of these different nitrogen species.”
Zakem said, “The long-standing hypothesis was that the locations of nitrification were controlled by the inhibition of light of these [nitrifying] microorganisms, so the microorganisms that carry out this process were restricted from the surface.”
“But instead of assuming that was true, the group examined the ecological interactions among these and other organisms in the surface ocean, letting the dynamics fall out naturally. ”
For this, scientists gathered microbial examples from the subtropical North Pacific and assessed them for digestion rates, efficiencies and plenitudes, and surveyed the physiological needs and imperatives of the distinctive nitrifying microorganisms by diminishing the organic many-sided quality of their digestion systems down to its hidden science and in this way theorizing a portion of the more principal obliges. They utilized this data to advise the elements of the nitrifying organisms in both a one-dimensional and three-dimensional biogeochemical display.
They found that by utilizing this structure, they could resolve the associations between these nitrifying chemoautotrophs and phytoplankton and thusly mimic the gathering of nitrite at the essential nitrite most extreme in the proper areas.
In the surface sea when inorganic nitrogen is a restricting component, phytoplankton and ammonium-oxidizing microorganisms have comparative capacities to obtain ammonium, but since phytoplankton require less nitrogen to develop and have a speedier development rate, they can outcompete the nitrifiers, barring them from the sunlit zone. Along these lines, they could give an environmental clarification to where nitrification occurs without relying on light restraint managing the area.
Looking at the basic physiologies of the nitrifiers uncovered that distinctions in digestion systems and cell size could represent the nitrite develop. The specialists found that the second step of the nitrification procedure that is done by the nitrite oxidizers requires more nitrogen for a similar measure of biomass being made by these living beings, implying that the alkali oxidizers can accomplish more with less and that there are fewer nitrite oxidizers than the smelling salts oxidizers.
The nitrite oxidizing microorganisms likewise have a higher surface to volume limitation than the littler and pervasive ammonium oxidizing organisms, making nitrogen take-up more troublesome. Here, nitrifiers can co-exist with phytoplankton since there’s more nitrogen available to them. Additionally, the deep mixed layer in the water can draw resources away from the phytoplankton, giving the nitrifiers a better chance at survival in the surface.
“This is an alternative explanation for why nitrite should accumulate,” Zakem says. “We have two reasons that point in the same direction. We can’t distinguish which one it is, but all of the observations are consistent with either of these two or some combination of both being the control.”
“There’s this long-standing hypothesis that the nitrifiers were inhibited by light and that’s why they only exist in the subsurface. We’re saying that maybe we have a more fundamental explanation: that this light inhibition does exist because we’ve observed it, but that’s a consequence of long-term exclusion from the surface.”
This study is of great significance as it provides evidence of how organisms’ individual traits affect competitive interactions among microbial populations and provide a direct control on nutrients’ distribution in the ocean.
The study is published online in the journal Nature Communications.
