Microscopic crystalline structures called metal-organic systems (MOFs) may give an approach to take care of one of the most serious issues in methane functionalization catalysis, a financially imperative chemical process.
The country’s shale gas production boom of ongoing years has driven numerous specialists to search for better approaches to functionalize methane — i.e., change it into something more significant. One such item could be methanol.
Now, scientists at the Northwestern University have demonstrated a new way to activate methane with MOFs. According to them, the study will showcase how outlining crystalline materials, specifically MOFs, will prompt arrangements of complex yet energizing openings.
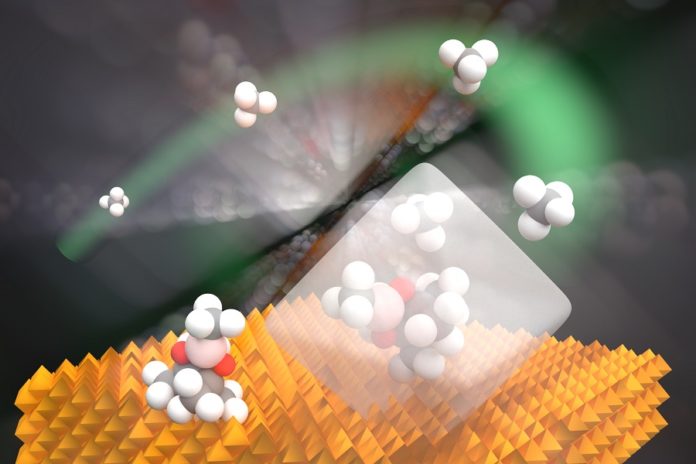
The Argonne-Northwestern team, in any case, has appeared out of the blue that MOFs can specifically create a particular boron-infused methane item by shape-selective catalysis, a generally utilized modern system for synthetics blend and hydrocarbon processing. Shape-specific catalysis can recognize particles that are somewhat unique in measure and may specifically form just a single wanted chemical product. Yet, for the system to work, the pore space of the impetus must be similar to the extent of the atoms associated with the reaction.
In MOFs, organic molecules and metal oxide clusters serve as the links and nodes, respectively. MOFs are attractive candidates for performing shape-selective catalysis because they are structurally tunable, noted lead author Xuan Zhang of Northwestern and his colleagues in the Nature Catalysis article. Unlike zeolites, they can be synthesized with pore and aperture sizes tailor-made for targeted molecules.
Laura Gagliardi, director of the Inorganometallic Catalyst Design Center, based at the University of Minnesota said, “Max Delferro and Omar Farha are excellent scientists who benefit from the infrastructure offered by the Inorganometallic Catalyst Design Center to perform state-of-the-art research that will advance the knowledge and the economy of our nation.”
Scientists showed that introduce a boron-based compound, in a process called borylation, and offer a promising route for methane activation under milder chemical conditions than would otherwise be possible.
They also observed that the borylation procedure yielding items that were both monoborylated (innovatively profitable) and bisborylated (undesired). Yet, by embeddings an iridium-based impetus (synthesized at Northwestern) inside the MOFs, scientists could deliver a response that shaped just the monoborylated item; the pores of the MOFs were too little for the bisborylated item to frame.
The Northwestern scientific experts additionally borylated the methane at the same time under an assortment of response conditions at Argonne’s High-Throughput Research Laboratory. The group at that point reported subtle elements of the iridium impetus’ oxidation state in X-beam assimilation tests at the Materials Research Collaborative Access Team’s (MR-CAT’s) x-ray beamline inside Argonne’s Advanced Photon Source (APS), a DOE Office of Science User Facility.
Delferro said, “We’re excited about the future for this chemistry. If we can do the same chemistry with iron, then we’re really in business.”
The study led by Delferro and Omar Farha, associate professor of chemistry at Northwestern University, has demonstrated a new way to activate methane with MOFs, as a result of their joint efforts in the Inorganometallic Catalyst Design Center, a DOE-funded Energy Frontier Research Center. They and seven co-authors recently published their method in Nature Catalysis.