Scientists at the UNIST have discovered a novel catalyst that generates electricity by causing a chemical reaction between hydrogen and oxygen in the air. The new catalysts with triple perovskites structure are relied upon to add to the commercialization of ‘reversible fuel cells’ since they indicate brilliant execution in the electrolysis of water.
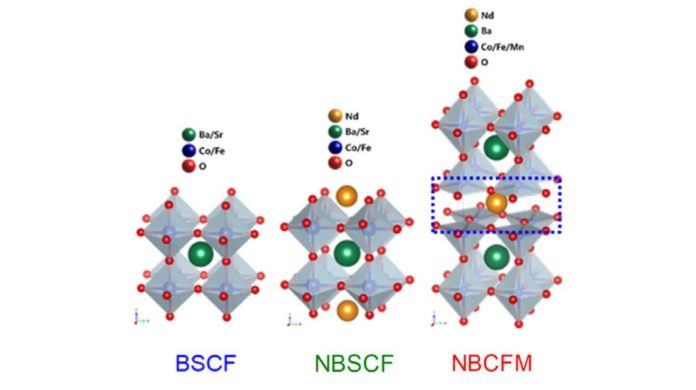
Scientists engineered an elite oxygen electrocatalyst in light of a triple perovskite, Nd1.5Ba1.5CoFeMnO9−δ (NBCFM), which indicates prevalent movement and solidness for oxygen cathode responses to single and twofold perovskites. The metal oxide which replaces platinum-based respectable metals. Perovskite is a natural inorganic-halogen (AMX₃) compound with a crystalline structure, for example, calcium titanate (CaTiO₃), which has been considered widely as a fuel cell catalyst. In the perovskite structure, A and M are metal cations and X is an anion containing a halide or an oxide.
This perovskite delivers a preferable catalytic activity and durability over single layer perovskite (BSCF) or double layer perovskite (NBSCF). This is attributed to the way that as the structure winds up confounded, the deformities increment and the reactivity turns out to be better.
The group likewise efficiently distinguished the connection between single-layer, double layer, and triple-layer perovskite structures and catalytic movement, and recognized the part of the defect structure in the oxygen-terminal catalytic action.
Scientists noted, “We will be able to develop new low-cost metal oxide catalysts based on our understanding of the catalytic sites obtained at this time.”
Professor Jun-Young Park from Sejong University said, “The catalytic material developed in this study will accelerate the commercialization of reversible fuel cells. We will be able to significantly improve existing performance by applying it to energy devices operating on a principle similar to that of fuel cells.”
This study published in Science Advances has been jointly led by Professor Jun-Young Park and Professor Sang Hoon Joo. Professor Taekjib Choi and Professor Young-Soo Seo from Sejong University, Professor Woo Seok Choi from Sungkyunkwan University, and Dr. Jun Yeon Hwang from Korea Institute of Science and Technology (KIST), and Dr. Kug-Seung Lee from Pohang Accelerator Laboratory have also partaken in this research.