A domino-type reaction, commonly used in various fields like synthetic organic chemistry and material engineering, is now being applied to redox chemistry. To extend the domino concept to redox chemistry, scientists at Hokkaido University designed and synthesized a dimeric quinodimethane (QD) with a nonplanar dithiin spacer.
The challenge in redox processes for achieving domino reactions is generating electrically charged species during electron transfer, which can hinder further responses due to electrostatic interactions.
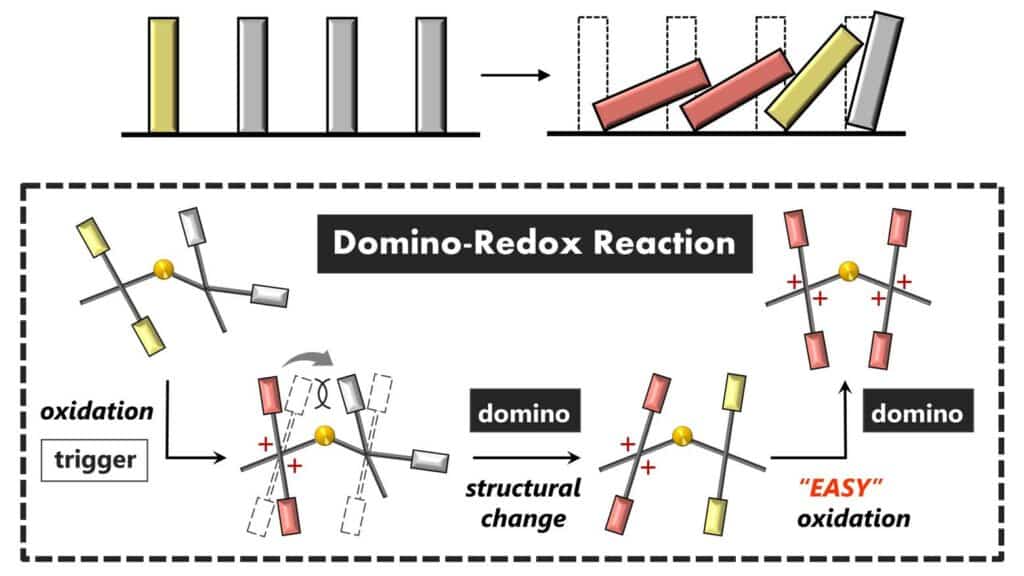
To address challenges in redox processes, the researchers developed a two-part molecule that undergoes a substantial structural change when one part transitions between its electrically neutral (reduced) and positively charged (oxidized) states. This change in structure influences the other part of the molecule, enhancing its likelihood of undergoing oxidation in a domino-like reaction.
The designed molecule comprises two sizable redox-active units linked by a flexible, non-planar structure of sulfur atoms. When one team loses electrons (oxidized), it gains two positive charges, serving as a trigger that causes the other part of the molecule to twist around the core. The altered electron state in this twisted form facilitates the oxidation process in the adjacent group, leading to a domino effect.
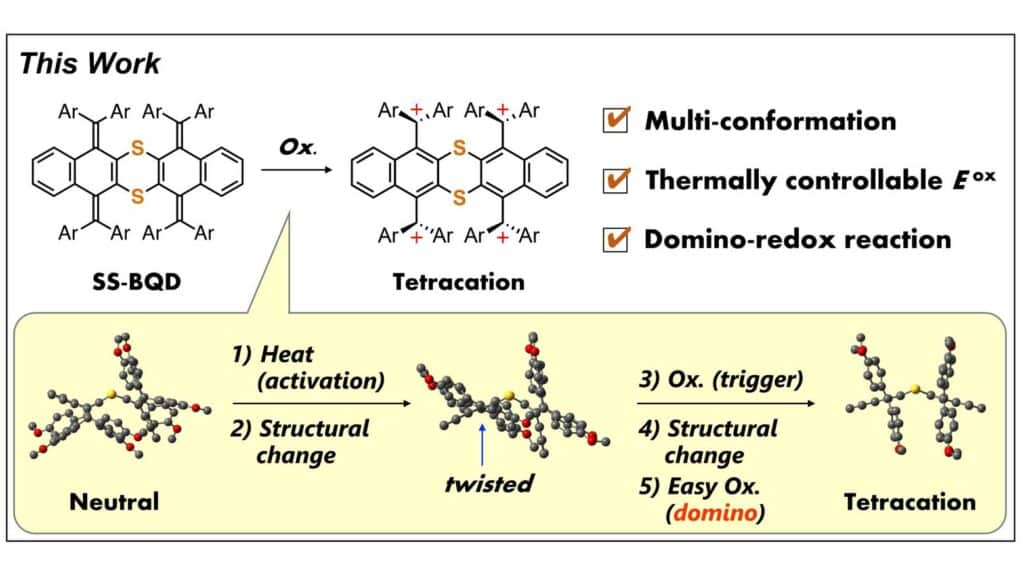
The initial triggering of the reaction can be induced by a temperature rise, providing a controllable method. While the effect has been demonstrated within a two-part molecule, the researchers envision its potential use to propagate wave-like redox transformations in larger molecules with multiple linked “domino” units.
Potential applications of this discovery include nano-scale components in chemical computation systems and sensors, as well as applications in new battery systems to support the transition to renewable energy technologies. However, practical applications are likely in the future.
Chemist Yusuke Ishigaki of the Hokkaido team said, “The control offered by heating and cooling could be used in many fields to make novel materials with switchable electronic properties, especially those involving multi-electron transfer.”
“It was very challenging, but also very satisfying, to demonstrate what nobody had achieved before, and we now hope to move into larger and more complex systems involving increased electron transfer.”
Journal Reference:
- Takashi Harimoto, et al. Domino-Redox Reaction Induced by An Electrochemically Triggered Conformational Change. Angewandte Chemie International Edition. DOI: 10.1002/anie.202316753
