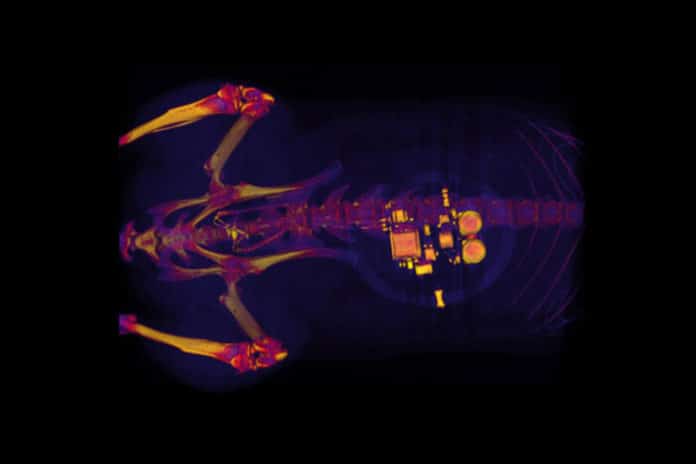
Scientists and engineers at the Washington University School of Medicine in St. Louis have developed a tiny, implantable device that can detect overactivity in the bladder and then use light from tiny, biointegrated LEDs to tamp down the urge to urinate.
The device works in laboratory rats and one day may help people who suffer incontinence or frequently feel the need to urinate.
Robert W. Gereau IV, Dr. Seymour, and Rose T. Brown, Professor of Anesthesiology at Washington University School of Medicine, said, “There definitely is a benefit to that sort of nerve stimulation. But there also are some off-target side effects that result from a lack of specificity with those older devices.”
Amid a minor surgery, they implanted a soft, stretchy belt-like device around the bladder. As the bladder fills and empties, the belt grows and contracts. The analysts likewise inject proteins called opsins into the animal’s bladders.
The opsins are conveyed by an infection that bound to nerve cells in the bladder, making those cells delicate to light signals. This enables the specialists to utilize optogenetics — the utilization of light to control cell conduct in living tissue — to actuate those cells.
Using blue-tooth communication to signal an external hand-held device, the scientists can read information in real time and, using a simple algorithm, detect when the bladder is full, when the animal has emptied its bladder, and when bladder emptying is occurring too frequently.
Gereau said, “When the bladder is emptying too often, the external device sends a signal that activates micro-LEDs on the bladder band device, and the lights then shine on sensory neurons in the bladder. This reduces the activity of the sensory neurons and restores normal bladder function.”
According to scientists, this strategy could work in people. Devices for individuals likely would be bigger than the ones utilized in rats and could be implanted without the medical procedure, utilizing catheters to put them through the urethra into the bladder.
John A. Rogers, the study’s other senior investigator and a professor of materials science and engineering at Northwestern said, “We’re excited about these results. This example brings together the key elements of an autonomous, implantable system that can operate in synchrony with the body to improve health: a precision biophysical sensor of organ activity; a noninvasive means to modulate that activity; a soft, battery-free module for wireless communication and control; and data analytics algorithms for closed-loop operation.”
Close-loop operation means- device can deliver the treatment just when it distinguishes an issue. At the point when the conduct is normalized, the micro- LEDs are killed, and treatment can be stopped.
Scientists are now expecting similar devices in larger animals. The specialists likewise trust the methodology could be utilized in different parts of the body — treating chronic pain, for instance, or utilizing light to animate cells in the pancreas to secrete insulin. One obstacle, notwithstanding, includes the viruses used to get light-sensitive proteins to bind to cells in organs.
Gereau said, “We don’t yet know whether we can achieve stable expression of the opsins using the viral approach and, more importantly, whether this will be safe over the long term. That issue needs to be tested in preclinical models and early clinical trials to make sure the strategy is completely safe.”
The new strategy is outlined in an article published Jan. 2 in the journal Nature.