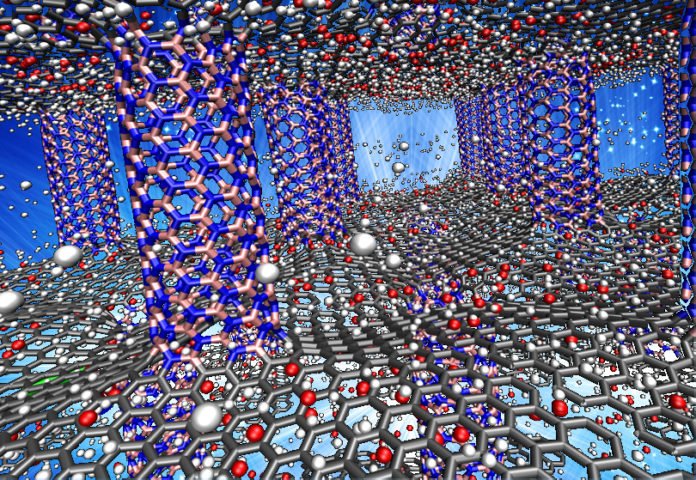
Rice University engineers have focused in on the ideal engineering for putting away hydrogen in “white graphene” nanomaterials — an outline like a Lilliputian high rise with “floors” of boron nitride sitting one on another and held decisively 5.2 angstroms separated by boron nitride columns.
Hydrogen’s primary drawbacks relate to portability, storage, and safety. Following months of calculations on two of Rice’s fastest supercomputers, scientists found the optimal architecture for storing hydrogen in boron nitride.
One type of the material, hexagonal boron nitride (hBN), comprises of particle thick sheets of boron and nitrogen and is now and again called white graphene in light of the fact that the iotas are separated precisely like carbon molecules in level sheets of graphene.
Study’s lead author, Rouzbeh Shahsavari said, “The motivation is to create an efficient material that can take up and hold a lot of hydrogen — both by volume and weight — and that can quickly and easily release that hydrogen when it’s needed.”
“The choice of material is important. Boron nitride has been shown to be better in terms of hydrogen absorption than pure graphene, carbon nanotubes or hybrids of graphene and boron nitride.”
“But the spacing and arrangement of hBN sheets and pillars are also critical. So we decided to perform an exhaustive search of all the possible geometries of hBN to see which worked best. We also expanded the calculations to include various temperatures, pressures, and dopants, trace elements that can be added to the boron nitride to enhance its hydrogen storage capacity.”
Scientists set up various “ab initio” tests, scientists reproductions that utilized first standards of material science. They conducted almost 4,000 ab initio calculations to try and find that sweet spot where the material and geometry go hand in hand and really work together to optimize hydrogen storage.
Unlike materials that store hydrogen through substance holding, boron nitride is a sorbent that holds hydrogen through physical securities, which are weaker than concoction securities. That is a favorable position with regards to getting hydrogen out of capacity since sorbent materials tend to release more effortlessly than their concoction cousins.
Shahsavari said, “the choice of boron nitride sheets or tubes and the corresponding spacing between them in the superstructure were the keys to maximizing capacity.”
“Without pillars, the sheets sit naturally one atop the other about 3 angstroms apart, and very few hydrogen atoms can penetrate that space. When the distance grew to 6 angstroms or more, the capacity also fell off. At 5.2 angstroms, there is a cooperative attraction from both the ceiling and floor and the hydrogen tends to clump in the middle. Conversely, models made of purely BN tubes — not sheets — had less storage capacity.”
The pure hBN tube-sheet structures could hold 8 weight percent of hydrogen. (Weight percent is a measure of concentration, similar to parts per million.) Physical experiments are needed to verify that capacity, but that the DOE’s ultimate target is 7.5 weight percent, and Shahsavari’s models suggest even more hydrogen can be stored in his structure if trace amounts of lithium are added to the hBN.
“Wrinkles form naturally in the sheets of pillared boron nitride because of the nature of the junctions between the columns and floors. In fact, this could also be advantageous because the wrinkles can provide toughness. If the material is placed under load or impact, that buckled shape can unbuckle easily without breaking. This could add to the material’s safety, which is a big concern in hydrogen storage devices.”
“Furthermore, the high thermal conductivity and flexibility of BN may provide additional opportunities to control the adsorption and release kinetics on-demand. For example, it may be possible to control release kinetics by applying an external voltage, heat or an electric field.”
The results appear in the journal Small.