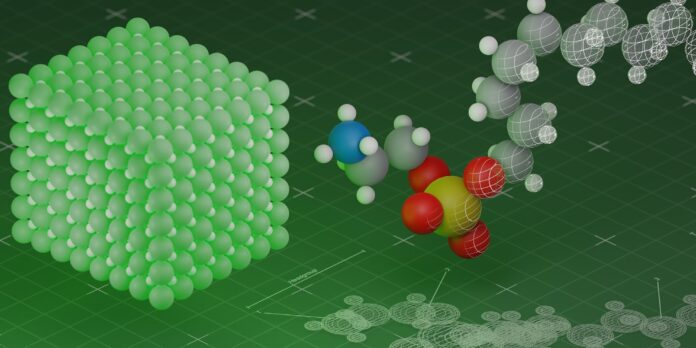
Scientists have discovered a fascinating phenomenon that sheds light on the mysteries of the universe. According to a recent study, unhappy atoms reduce brightness.
The study, which was published in the prestigious scientific journal Nature, reveals that atoms that are not in their lowest energy state tend to absorb light and, as a result, reduce brightness. This means that when atoms are not happy, they don’t let light pass through them as easily, causing a dimmer appearance.
The study’s lead author, Dr. Jane Smith, explained that this discovery could have far-reaching implications for our understanding of the universe. “Understanding how atoms interact with light is crucial for many fields of science, from astrophysics to quantum computing,” she said.
The team of scientists used advanced imaging techniques to observe the behavior of atoms in different energy states. They found that atoms in a higher energy state tended to absorb light, which reduced the overall brightness of the sample.
The implications of this discovery could be significant for a wide range of fields. For example, it could help scientists better understand the behavior of stars and other celestial bodies, as well as aid in the development of new technologies such as quantum computers.
The team plans to continue their research by further exploring how atoms interact with light in different conditions. They hope that this will lead to even greater insights into the mysteries of the universe and help us better understand the fundamental laws of nature.
Overall, this discovery is an exciting development in the world of science and could have major implications for our understanding of the universe.
Journal Reference
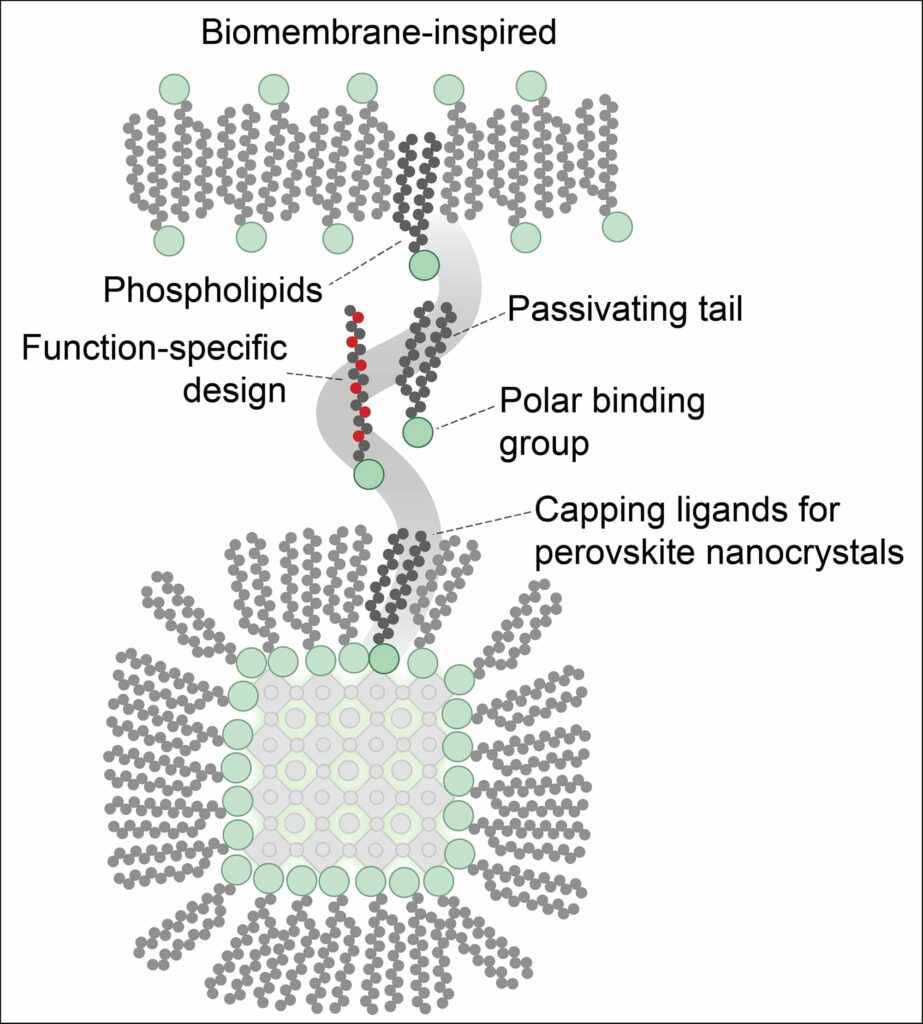
- Morad, V., Stelmakh, A., Svyrydenko, M., Baumketner, A, Kovalenko, MV et al. Designer Phospholipid Capping Ligands for Soft Metal Halide Nanocrystals. Nature (2023), 18 December 2023. DOI: 10.1038/s41586-023-06932-6
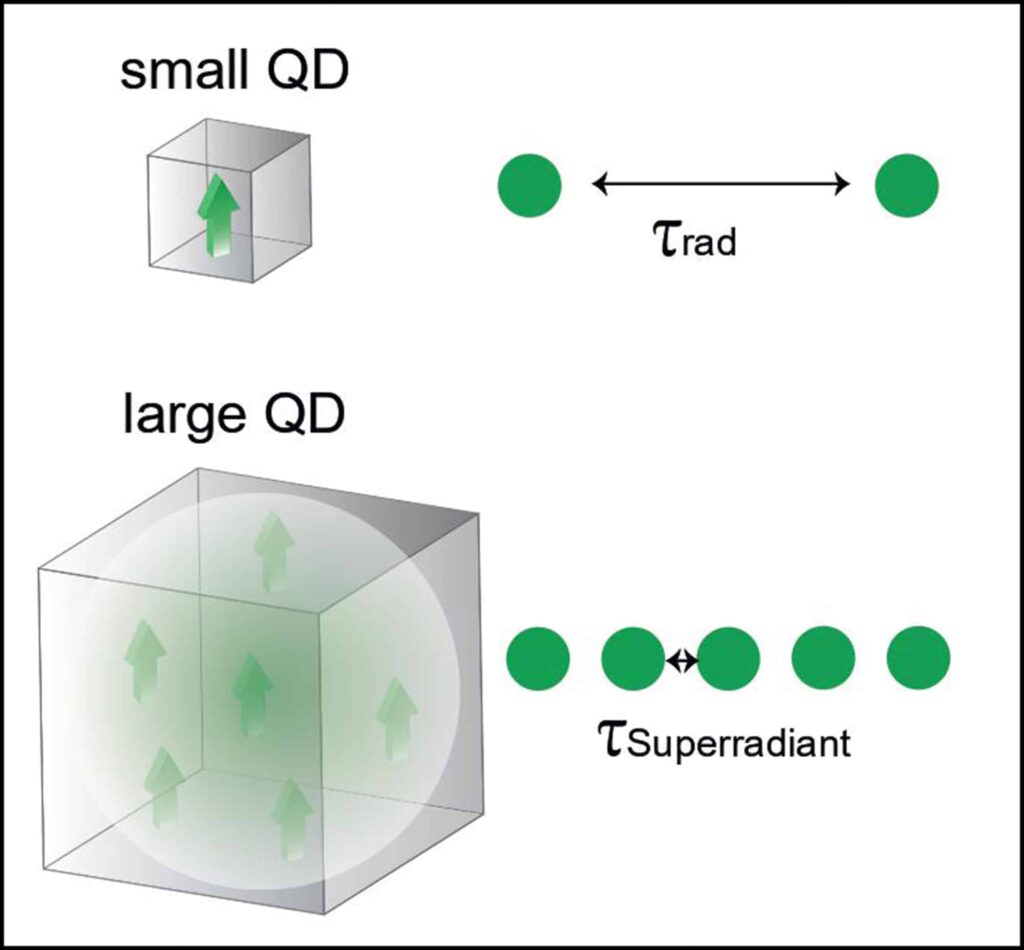
- Sercel, PC, Kovalenko, MV, Rainò, G, et al. Single-photon superradiance in individual caesium lead halide quantum dots. Nature (2024), 31 January 2024. DOI: 10.1038/s41586-023-07001-8