By extremely diminishing the impacts of antibiotics, the development of organized networks of bacterial cells known as biofilms can be dangerous amid medical procedures and in urinary tract infections. Yale specialists have quite recently come close to seeing how these biofilms create and conceivably how to stop them.
Biofilms shape when bacterial cells gather and develop structures that bond them in a gooey substance. This glue can protect the cells from the outside world and enable them to frame complex quasi-organisms. Biofilms can be found all over, including unwashed shower stalls or the surfaces of lakes.
Since the protective shell can keep potential medications, biofilms are at their most risky when they attack human cells or frame on sutures and catheters utilized in medical procedures. In American hospitals alone, a great many passing are attributed to biofilm-related surgical site contaminations and urinary tract diseases.
Andre Levchenko, senior author of the study, said, “Biofilms are a huge medical problem because they are something that makes bacterial infections very difficult to deal with.”
Battling biofilms has been especially troublesome on the grounds that it hasn’t been surely knew how bacteria cells make the progress from carrying on separately to existing in collective structures. Be that as it may, the analysts in the Levchenko lab, working with associates at the University of California-San Diego, have recently found a key component for biofilm arrangement that additionally gives an approach to ponder this procedure in a controlled and reproducible way.
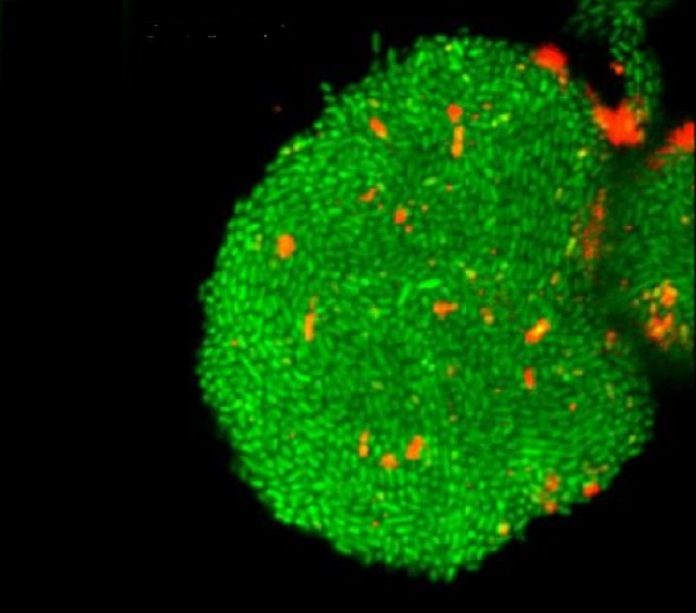
Scientists outlined and developed microfluidic devices and novel gels that housed uropathogenic E. coli cells, which are regularly the reason for urinary tract contaminations. These gadgets mimic the earth inside human cells that host the invading bacteria amid diseases.
The researchers found that the bacterial provinces would develop to the point where they would be crushed by either the walls of the chamber, the fibers, or the gel. This self-created pressure was itself a trigger of the biofilm formation.
Levchenko, the John C. Malone Professor of Biomedical Engineering, said, “This was very surprising, but we saw all the things you would expect from a biofilm. The cells produced the biofilm components and suddenly became very antibiotic-resistant. And all of that was accompanied by an indication that the cells were under biological stress and the stress was coming from this mechanical interaction with the environment.”
“With this discovery, researchers can use various devices that mimic other cellular environments and explore biofilm formation under countless environments and circumstances. They can also use the devices introduced in this study to produce biofilms rapidly, precisely, and in high numbers in a simple, inexpensive, and reproducible way. This would allow screening drugs that could potentially breach the protective layer of the biofilms and break it down.”
“Having a disease model like this is a must when you want to do these kinds of drug-screening experiments. We can now grow biofilms in specific shapes and specific locations in a completely predictable way.”
Scientists have published their study in the journal Nature Communications.