Scientists financed by the National Eye Institute (NEI) have switched congenital visual blindness in mice by changing supportive cells in the retina called Müller glia into rod photoreceptors. The discoveries propel efforts toward regenerative treatments for blinding diseases, for example, age-related macular degeneration and retinitis pigmentosa.
Researchers have since quite a while ago concentrated the regenerative capability of Müller glia on the grounds that in different species, for example, zebrafish, they separate in light of damage and can transform into photoreceptors and other retinal neurons. The zebrafish would thus be able to recapture vision after serious retinal damage. In the lab, be that as it may, researchers can coax mammalian Müller glia to carry on more as they do in the fish. In any case, it requires harming the tissue.
Bo Chen, Ph.D., associate professor of ophthalmology and director of the Ocular Stem Cell Program at the Icahn School of Medicine at Mount Sinai, New York said, “From a practical standpoint, if you’re trying to regenerate the retina to restore a person’s vision, it is counterproductive to injure it first to activate the Müller glia. We wanted to see if we could program Müller glia to become rod photoreceptors in a living mouse without having to injure its retina.”
To do so, scientists spurred Müller glia in normal mice to divide by injecting their eyes with a gene to turn on a protein called beta-catenin. Weeks after the fact, they infused the mice’s eyes with factors that energized the recently separated cells to form into rod photoreceptors.
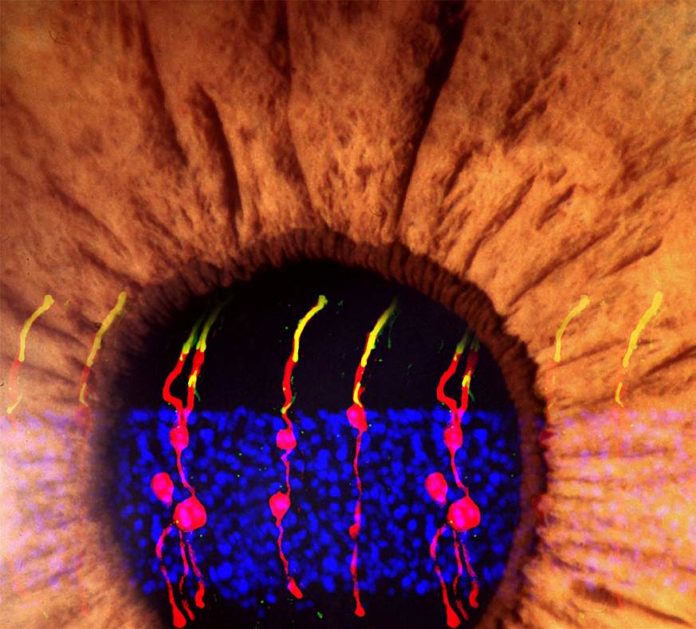
The specialist’s utilized microscopy to visually track the recently framed cells. They found that the recently framed rod photoreceptors appeared to be fundamentally no unique from genuine photoreceptors. Likewise, synaptic structures that enable the rods to speak with different kinds of neurons inside the retina had additionally shaped.
To determine if the Müller glia-inferred bar photoreceptors were useful, they tried the treatment in mice with inborn visual deficiency, which implied that they were conceived without practical bar photoreceptors.
In the treated mice that were born blind, Müller glia-inferred rods grew similarly as viable as they had in normal mice. Practically, they affirmed that the recently framed bars were interacting with different kinds of retinal neurons crosswise over neurotransmitters.
Moreover, light reactions recorded from retinal ganglion cells—neurons that carry signals from photoreceptors to the brain—and measurements of brain activity confirmed that the newly-formed rods were, in fact, integrating into the visual pathway circuitry, from the retina to the primary visual cortex in the brain.
Chen’s lab is conducting behavioral studies to determine whether the mice have regained the ability to perform visual tasks such as a water maze task. Chen also plans to see if the technique works on a cultured human retinal tissue.
A report of the findings appears online in Nature.