The current outbreak of the pandemic coronavirus demands its rapid, convenient, and large-scale diagnosis to downregulate its spread within as well as across the communities. But, the reliability, reproducibility, and selectivity of the majority of such diagnostic tests fail when they are tested either to a viral load at its early representation or to a viral gene mutated during its current spread.
In a new study, scientists from the University of Maryland School of Medicine (UMSOM) have devised an experimental diagnostic test for COVID-19 that can visually detect the presence of the virus in 10 minutes.
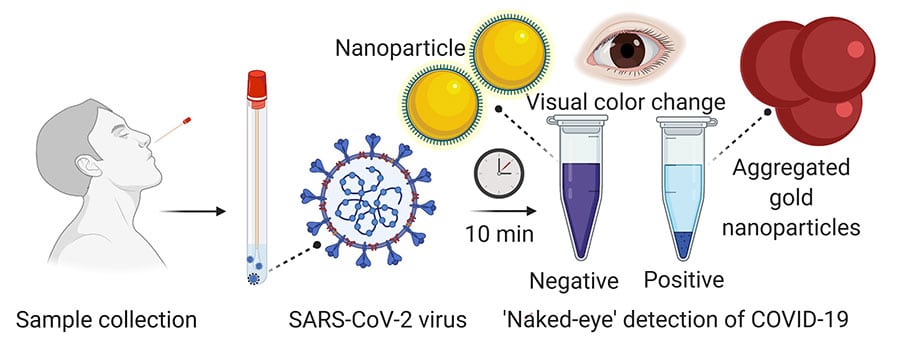
The test uses a simple assay containing plasmonic gold nanoparticles to detect a color change when the virus is present. And fascinatingly, the test does not need any additional lab techniques for analysis.
When a nasal swab or saliva sample is acquired from a patient, the RNA is separated from the sample by means of a simple procedure that takes around 10 minutes. The test utilizes an exceptionally explicit particle appended to the gold nanoparticles to identify a specific protein.
This protein is a part of the genetic sequence that is unique to the novel coronavirus. When the biosensor binds to the virus’s gene sequence, the gold nanoparticles react by turning the liquid reagent from purple to blue.
Study leader Dipanjan Pan, Ph.D., Professor of Diagnostic Radiology and Nuclear Medicine and Pediatrics at the UMSOM, said, “Based on our preliminary results, we believe this promising new test may detect RNA material from the virus as early as the first day of infection. Additional studies are needed, however, to confirm whether this is indeed the case.”
Study co-author Matthew Frieman, Ph.D., Associate Professor of Microbiology and Immunology at UMSOM, said, “This RNA-based test appears to be very promising in terms of detecting the virus. The innovative approach provides results without the need for a sophisticated laboratory facility.”
Dr. Pan created a company called VitruVian Bio to develop the test for commercial applications. He plans to have a pre-submission meeting with the U.S. Food and Drug Administration (FDA) within the next month to discuss requirements for getting an emergency use authorization for the test. New FDA policy allows for the marketing of COVID-19 tests without requiring them to go through the usual approval or clearance process. These tests do, however, need to meet specific validation testing requirements to ensure that they provide reliable results.
Journal Reference:
- Parikshit Moitra et al., Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. DOI: 10.1021/acsnano.0c03822