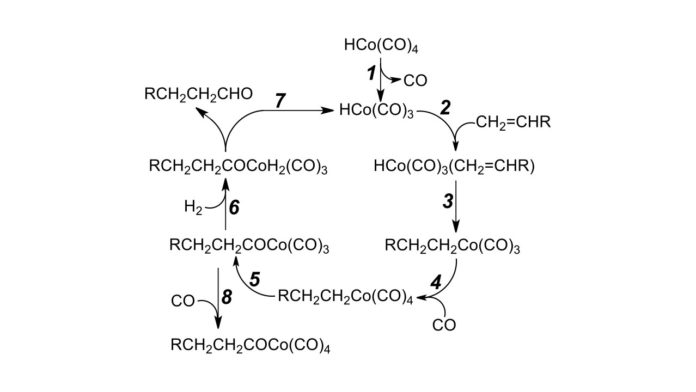
Hydroformylation is the catalytic reaction that converts alkenes, carbon monoxide, and hydrogen into more complex organic products, like plasticizers–a substance added to produce flexibility and to reduce brittleness–and cleaning detergents. Hydroformylation reactions are applied at a massive scale in the chemical industry to transform olefins into aldehydes. The original catalysts were neutral cobalt complexes.
Almost 75 percent of industries use rhodium-based catalysts because of the low-pressure technologies and cheaper-to-build facilities.
In a new study, LSU chemistry professor emeritus George Stanley and fellow LSU researchers from the Department of Chemistry and the Department of Biological Sciences discovered a new cationic cobalt bisphosphine hydroformylation catalyst system that is highly active and extremely robust.
According to scientists, not exclusively can cobalt-based catalysts make more- – and better forms – of certain aldehyde products, but the cost of rhodium is high in comparison.
Stanley said, “A cationic cobalt bisphosphine catalyst is only about 20 times slower than the best rhodium catalysts, despite being 10,000 times less expensive.”
“About 25 percent of products produced by hydroformylation require high-pressure cobalt or rhodium technologies. This new cationic cobalt bisphosphine technology offers a far more energy-efficient catalyst that can operate at medium pressures for these reactions.”
However, this new catalyst has low selectivity to the generally desired linear aldehyde product for simple alkenes. Yet, it has excellent activity and selectivity for internal branched alkenes that are difficult to hydroformylate.
Stanley said, “this is the first major discovery in hydroformylation in at least 50 years.”
“What excites me the most is to have a discovery that could have real-life practical applications. Coming up with a catalyst that is very energy efficient, very green, that can be used on the large-scale, industrial side of things is the dream of every chemist.”
The study is published in journal Science.