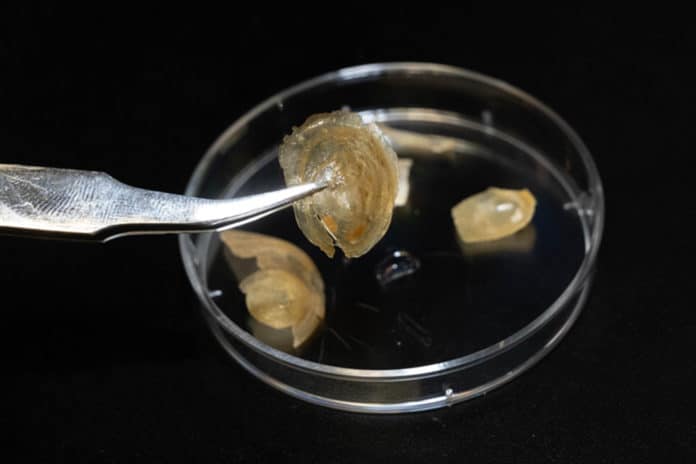
Why the protective cover of the brachiopod Discinisca tenuis becomes incredibly soft in water and gets hard again in the air?
A new study by an international research team with the Paul Scherrer Institute PSI has revealed the reason.
The brachiopod Discinisca tenuis’s shell is enriched with a mineral that protects it from harmful environmental influences. When the shell is in water, it changes its structure in a material. As a result, the shell becomes so flexible that it can even be folded up without breaking.
Scientists wanted to know how this transformation happens.
Fabio Nudelman, a materials chemist currently at the School of Chemistry, the University of Edinburgh in Scotland, said, “In its composition, the shell resembles bone. But bone doesn’t change its structure when it gets wet. The same goes for clams: If the animals need to adapt the properties of their shell to different environmental conditions, they normally have to rework the material in a lengthy and energetically costly process by resorbing and redistributing minerals. It doesn’t work simply through the absorption of water.”
Scientists used a cryo-tomography technique to examine the material as if under a very high-resolution microscope and at extremely low temperatures.
Johannes Ihli, a PSI researcher at SLS, said, “At room temperature, it would not have been possible since the high-energy X-ray light would immediately alter the sensitive shell structure.”
They found the shell, which is more than half a millimeter thick, consists of a hybrid material. It mainly consists of inorganic minerals in which organic polymers made from proteins and sugars are embedded.
The mineral that constitutes the main component of the shell is a type of fluoroapatite – similar to the material that makes up the enamel of our teeth.
Ihli said, “Tiny nanocrystals of this material are arranged in layers that can be compared with brick walls. In this analogy, the bricks are the nanocrystals, and the mortar between the bricks consists of organic molecules such as chitin and proteins.”
“This ‘mortar’ can absorb large amounts of water, causing it to swell up. Through the storage of water, it changes its structure: It becomes soft, and the bricks become movable with respect to each other.”
“Then water acts like a lubricant between the individual nanocrystals. The crystals can then slip against each other. Through this movement, the shell becomes flexible.”
The observation also revealed a network of pores in the shell. These pores effectively guide water inside and rapidly distributing it throughout the material.
Scientists speculate that the shell effectively guided water inside and rapidly distributed it throughout the material.
Nudelman said, “This could prevent damage to the shell and thus be a key to the animals’ survival. The phenomenon may even be more widespread than suspected: “We don’t know how many other animal species there might be that have this kind of property.”
Journal Reference:
- J. Ihli, A.S. Schenk et al. Mechanical Adaptation of Brachiopod Shells Via Hydration-Induced Structural Changes. Nature Communications, 10 September 2021 (online). DOI: 10.1038/s41467-021-25613-4